MHC class II

MHC class II molecules are a class of major histocompatibility complex (MHC) molecules normally found only on antigen-presenting cells such as dendritic cells, mononuclear phagocytes, some endothelial cells, thymic epithelial cells, and B cells. These cells are important in initiating immune responses.
The antigens presented by class II peptides are derived from extracellular proteins (not cytosolic as in MHC class I).
Loading of a MHC class II molecule occurs by phagocytosis; extracellular proteins are endocytosed, digested in lysosomes, and the resulting epitopic peptide fragments are loaded onto MHC class II molecules prior to their migration to the cell surface.
In humans, the HLAs corresponding to MHC class II are HLA-DP, HLA-DM, HLA-DOA, HLA-DOB, HLA-DQ, and HLA-DR.
Structure
Like MHC class I molecules, class II molecules are also heterodimers, but in this case consist of two homogenous peptides, an α and β chain, both of which are encoded in the MHC.[1] The subdesignation α1, α2, etc. refers to separate domains within the HLA gene; each domain is usually encoded by a different exon within the gene, and some genes have further domains that encode leader sequences, transmembrane sequences, etc.
Because the antigen-binding groove of MHC class II molecules is open at both ends while the corresponding groove on class I molecules is closed at each end, the antigens presented by MHC class II molecules are longer, generally between 15 and 24 amino acid residues long.
Expression
These molecules are constitutively expressed in professional, immune antigen presenting cells, but may also be induced on other cells by interferon γ.[2]
MHC class II is also expressed on group 3 innate lymphoid cells.
Reaction to bacteria
Because class II MHC is loaded with extracellular proteins, it is mainly concerned with presentation of extracellular pathogens (for example, bacteria that might be infecting a wound or the blood). Class II molecules interact mainly with immune cells, like the T helper cell (TCD4+) . The helper T cells then help to trigger an appropriate immune response which may include localized inflammation and swelling due to recruitment of phagocytes or may lead to a full-force antibody immune response due to activation of B cells.
Synthesis
During synthesis of class II MHC in the endoplasmic reticulum, the α and β chains are produced and complexed with a special polypeptide known as the invariant chain. The nascent MHC class II protein in the rough ER has its peptide-binding cleft blocked by the invariant chain (Ii; a trimer) to prevent it from binding cellular peptides or peptides from the endogenous pathway (such as those that would be loaded onto class I MHC).
The invariant chain also facilitates the export of class II MHC from the ER to the golgi, followed by fusion with a late endosome containing endocytosed, degraded proteins. The invariant chain is then broken down in stages by proteases called cathepsins, leaving only a small fragment known as CLIP which maintains blockage of the peptide binding cleft on the MHC molecule. An MHC class II-like structure, HLA-DM, facilitates CLIP removal and allows the binding of peptides with higher affinities. The stable class II MHC is then presented on the cell surface.
Genes
| Alpha | Beta | |
| HLA-DM | HLA-DMA | HLA-DMB |
| HLA-DO | HLA-DOA | HLA-DOB |
| HLA-DP | HLA-DPA1 | HLA-DPB1 |
| HLA-DQ | HLA-DQA1, HLA-DQA2 | HLA-DQB1, HLA-DQB2 |
| HLA-DR | HLA-DRA | HLA-DRB1, HLA-DRB3, HLA-DRB4, HLA-DRB5 |
Pathways controlling MHC class II antigen presentation
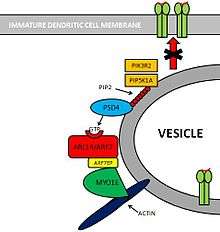
Pathway: PSD4 - ARL14/ARF7 - MYO1E
Molecules involved
Several molecules are involved in this pathway.[3]
- PIK3R2[4] and PIP5K1A[5] are two kinases that create substrates for PSD4.
- PSD4[6][7] (Pleckstrin and Sec7 Domain containing 4) is a GEF (Guanine Exchange Factor) that loads ARL14/ARF7 with GTP.
- ARL14/ARF7[8] is a Small GTPase protein that is selectively expressed in immune cells. This protein is localized within MCH-II compartments in immature Dendritic Cells.
- ARF7EP[9] is an effector of ARL14/ARF7 that interacts with MYO1E.
- MYO1E[10] is a protein that controls MHC-II compartments with an actin-based mechanism.
Pathway
PIK3R2 and PIP5K1A are two Kinases that phosphorylate Phosphatidylinositol (PIP) providing PSD4 with substrates for its GTP loading ability. PSD4 as a Guanine Exchange Factor, loads ARL14/ARF7 with GTP. Subsequently, ARF7EP interacts with MYO1E which binds itself to Actin myofibers. Altogether, this complex contributes to maintain MHC-II loaded vesicles within the Immature Dendritic Cell, impeding its translocation to the cell membrane.
See also
References
- ↑ "Histocompatibility". Retrieved 2009-01-21.
- ↑ "Genetic control of MHC class II expression.". Cell. 109 Suppl: S21–33. Apr 2002. doi:10.1016/s0092-8674(02)00696-7. PMID 11983150.
- ↑ "A Genome-wide multidimensional RNAi screen reveals pathways controlling MHC class II antigen presentation". Cell. 145 (2): 268–83. 2011. doi:10.1016/j.cell.2011.03.023.
- ↑ PIK3R2 phosphoinositide-3-kinase, regulatory subunit 2 (beta) [Homo sapiens (human)] - Gene - NCBI
- ↑ PIP5K1A phosphatidylinositol-4-phosphate 5-kinase, type I, alpha [Homo sapiens (human)] - Gene - NCBI
- ↑ PSD4 pleckstrin and Sec7 domain containing 4 [Homo sapiens (human)] - Gene - NCBI
- ↑ "ARF6 controls post-endocytic recycling through its downstream exocyst complex effector". J Cell Biol. 163 (5): 1111–21. 2003. doi:10.1083/jcb.200305029. PMC 2173613
 . PMID 14662749.
. PMID 14662749. - ↑ ARL14 ADP-ribosylation factor-like 14 [Homo sapiens (human)] - Gene - NCBI
- ↑ ARL14EP ADP-ribosylation factor-like 14 effector protein [Homo sapiens (human)] - Gene - NCBI
- ↑ MYO1E myosin IE [Homo sapiens (human)] - Gene - NCBI
External links
- Histocompatibility Antigens Class II at the US National Library of Medicine Medical Subject Headings (MeSH)
- MHC Class II Genes at the US National Library of Medicine Medical Subject Headings (MeSH)
- http://www.merck.com/mmpe/sec13/ch163/ch163a.html