MAPK/ERK pathway
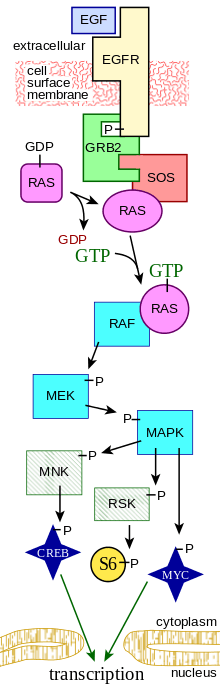
The MAPK/ERK pathway (also known as the Ras-Raf-MEK-ERK pathway) is a chain of proteins in the cell that communicates a signal from a receptor on the surface of the cell to the DNA in the nucleus of the cell.
The signal starts when a signaling molecule binds to the receptor on the cell surface and ends when the DNA in the nucleus expresses a protein and produces some change in the cell, such as cell division. The pathway includes many proteins, including MAPK (mitogen-activated protein kinases, originally called ERK, extracellular signal-regulated kinases), which communicate by adding phosphate groups to a neighboring protein, which acts as an "on" or "off" switch.
When one of the proteins in the pathway is mutated, it can become stuck in the "on" or "off" position, which is a necessary step in the development of many cancers. Components of the MAPK/ERK pathway were discovered when they were found in cancer cells. Drugs that reverse the "on" or "off" switch are being investigated as cancer treatments.[1]
Background
Overall, the extracellular mitogen binds to the membrane receptor. This allows Ras (a GTPase) to swap its GDP for a GTP. It can now activate MAP3K (e.g., Raf), which activates MAP2K, which activates MAPK. MAPK can now activate a transcription factor, such as Myc. Receptor-linked tyrosine kinases such as the epidermal growth factor receptor (EGFR) are activated by extracellular ligands. Binding of epidermal growth factor (EGF) to the EGFR activates the tyrosine kinase activity of the cytoplasmic domain of the receptor. The EGFR becomes phosphorylated on tyrosine residues. Docking proteins such as GRB2 contain an SH2 domain that binds to the phosphotyrosine residues of the activated receptor.[2] GRB2 binds to the guanine nucleotide exchange factor SOS by way of the two SH3 domains of GRB2. When the GRB2-SOS complex docks to phosphorylated EGFR, SOS becomes activated.[3] Activated SOS then promotes the removal of GDP from a member of the Ras subfamily (most notably H-Ras or K-Ras). Ras can then bind GTP and become active.
Apart from EGFR, other cell surface receptors that can activate this pathway via GRB2 include Trk A/B, Fibroblast growth factor receptor (FGFR) and PDGFR.
Kinase cascade
Activated Ras activates the protein kinase activity of RAF kinase.[4] RAF kinase phosphorylates and activates MEK (MEK1 and MEK2). MEK phosphorylates and activates a mitogen-activated protein kinase (MAPK).
RAF, and ERK (also known as MAPK) are both serine/threonine-selective protein kinases. MEK is a serine/tyrosine/threonine kinase.
In the technical sense, RAF, MEK, and MAPK are all mitogen-activated kinases, as is MNK (see below). MAPK was originally called "extracellular signal-regulated kinases" (ERKs) and "microtubule associated protein kinase" (MAPK). One of the first proteins known to be phosphorylated by ERK was a microtubule-associated protein (MAP). As discussed below, many additional targets for phosphorylation by MAPK were later found, and the protein was renamed "mitogen-activated protein kinase" (MAPK). The series of kinases from RAF to MEK to MAPK is an example of a protein kinase cascade. Such series of kinases provide opportunities for feedback regulation and signal amplification.
Regulation of translation and transcription
Three of the many proteins that are phosphorylated by MAPK are shown in the Figure. One effect of MAPK activation is to alter the translation of mRNA to proteins. MAPK phosphorylates 40S ribosomal protein S6 kinase (RSK). This activates RSK, which, in turn, phosphorylates ribosomal protein S6.[5] Mitogen-activated protein kinases that phosphorylate ribosomal protein S6 were the first to be isolated.[4]
MAPK regulates the activities of several transcription factors. MAPK can phosphorylate C-myc. MAPK phosphorylates and activates MNK, which, in turn, phosphorylates CREB. MAPK also regulates the transcription of the C-Fos gene. By altering the levels and activities of transcription factors, MAPK leads to altered transcription of genes that are important for the cell cycle.
The 22q11, 1q42, and 19p13 genes are associated with schizophrenia, schizoaffective, bipolar, and migraines by affecting the ERK pathway.

Clinical significance
Uncontrolled growth is a necessary step for the development of all cancers.[6] In many cancers (e.g. melanoma), a defect in the MAP/ERK pathway leads to that uncontrolled growth. Many compounds can inhibit steps in the MAP/ERK pathway, and therefore are potential drugs for treating cancer,[7][8][9] [10][11] e.g., Hodgkin disease.[12]
The first drug licensed to act on this pathway is sorafenib — a Raf kinase inhibitor.
Other Raf inhibitors: SB590885, PLX4720, XL281, RAF265, encorafenib, dabrafenib, vemurafenib.[11]
Some MEK inhibitors: cobimetinib, CI-1040, PD035901, Binimetinib (MEK162), selumetinib,[11] Trametinib(GSK1120212)[13]
RAF-ERK pathway is also involved in the pathophysiology of Noonan's Syndrome, a polymalformative disease, where Simvastatin was proposed as a way to improve CNS-cognitive symptoms of the disorder.
Protein microarray analysis can be used to detect subtle changes in protein activity in signaling pathways.[14] The developmental syndromes caused by germline mutations in genes that alter the RAS components of the MAP/ERK signal transduction pathway are called RASopathies.
See also
References
- ↑ Orton RJ, Sturm OE, Vyshemirsky V, Calder M, Gilbert DR, Kolch W (Dec 2005). "Computational modelling of the receptor-tyrosine-kinase-activated MAPK pathway". The Biochemical Journal. 392 (Pt 2): 249–61. doi:10.1042/BJ20050908. PMC 1316260
 . PMID 16293107.
. PMID 16293107. - ↑ Schulze WX, Deng L, Mann M (2005). "Phosphotyrosine interactome of the ErbB-receptor kinase family". Molecular systems biology. 1 (1): 2005.0008. doi:10.1038/msb4100012. PMC 1681463
 . PMID 16729043.
. PMID 16729043. - ↑ Zarich N, Oliva JL, Martínez N, et al. (Aug 2006). "Grb2 is a negative modulator of the intrinsic Ras-GEF activity of hSos1". Molecular Biology of the Cell. 17 (8): 3591–7. doi:10.1091/mbc.E05-12-1104. PMC 1525251
 . PMID 16760435.
. PMID 16760435. - 1 2 Avruch J, Khokhlatchev A, Kyriakis JM, et al. (2001). "Ras activation of the Raf kinase: tyrosine kinase recruitment of the MAP kinase cascade". Recent Progress in Hormone Research. 56 (1): 127–55. doi:10.1210/rp.56.1.127. PMID 11237210.
- ↑ Pende M, Um SH, Mieulet V, et al. (Apr 2004). "S6K1,(-/-)/S6K2(-/-) mice exhibit perinatal lethality and rapamycin-sensitive 5'-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway". Molecular and Cellular Biology. 24 (8): 3112–24. doi:10.1128/MCB.24.8.3112-3124.2004. PMC 381608
 . PMID 15060135.
. PMID 15060135. - ↑ Downward J (2003). "Targeting RAS signalling pathways in cancer therapy". Nature Reviews Cancer. 3 (1): 11–22. doi:10.1038/nrc969. PMID 12509763.
- ↑ Hilger RA, Scheulen ME, Strumberg D (December 2002). "The Ras-Raf-MEK-ERK pathway in the treatment of cancer" (PDF). Onkologie. 25 (6): 511–8. doi:10.1159/000068621. PMID 12566895.
- ↑ Sebolt-Leopold JS (June 2008). "Advances in the development of cancer therapeutics directed against the RAS-mitogen-activated protein kinase pathway". Clin. Cancer Res. 14 (12): 3651–6. doi:10.1158/1078-0432.CCR-08-0333. PMID 18559577.
- ↑ Hoshino R, Chatani Y, Yamori T, et al. (January 1999). "Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors". Oncogene. 18 (3): 813–22. doi:10.1038/sj.onc.1202367. PMID 9989833.
- ↑ McCubrey JA, Steelman LS, Chappell WH, et al. (August 2007). "Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance". Biochim. Biophys. Acta. 1773 (8): 1263–84. doi:10.1016/j.bbamcr.2006.10.001. PMC 2696318
 . PMID 17126425.
. PMID 17126425. - 1 2 3 Kwong-Kwok Wong (2009). "Recent Developments in Anti-Cancer Agents Targeting the Ras/Raf/ MEK/ERK Pathway" (PDF).
- ↑ Zheng B, Fiumara P, Li YV, et al. (August 2003). "MEK/ERK pathway is aberrantly active in Hodgkin disease: a signaling pathway shared by CD30, CD40, and RANK that regulates cell proliferation and survival". Blood. 102 (3): 1019–27. doi:10.1182/blood-2002-11-3507. PMID 12689928.
- ↑ http://clinicaltrialsfeeds.org/clinical-trials/show/NCT01037127
- ↑ Calvert VS, Tang Y, Boveia V, Wulfkuhle J, Schutz-Geschwender A, Oliver DM, Liotta LA, Petricoin EF (2004). "Development of Multiplexed Protein Profiling and Detection Using Near Infrared Detection of Reverse-Phase Protein Microarrays" (PDF). Clinical Proteomics Journal. 1 (1): 81–89. doi:10.1385/CP:1:1:081.
External links
- MAP Kinase Resource .
- Kyoto Encyclopedia of Genes and Genomes — MAPK pathway
- MAP Kinase Signaling System at the US National Library of Medicine Medical Subject Headings (MeSH)