Lysophosphatidylcholine
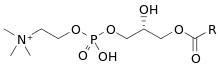
Lysophosphatidylcholines (LPC, lysoPC), also called lysolecithins, are a class of chemical compounds which are derived from phosphatidylcholines.
Overview
They result from partial hydrolysis of phosphatidylcholines, which removes one of the fatty acid groups. The hydrolysis is generally the result of the enzymatic action of phospholipase A2.[1] Among other properties, they activate endothelial cells during early atherosclerosis[2] and stimulate phagocyte recruitment when released by apoptotic cells.[3] Moreover, LPCs can be used in the lab to cause demyelination of brain slices, to mimic the effects of demyelinating diseases such as multiple sclerosis. Further, they are known to stimulate phagocytosis of the myelin sheath and can change the surface properties of erythrocytes.[4] LPC-induced demyelination is thought to occur through the actions of recruited macrophages and microglia which phagocytose nearby myelin. Invading T cells are also thought to mediate this process. Bacteria such as Legionella pneumophila utilize phospholipase A2 end-products ( fatty acids and lysophospholipids) to cause host cell (macrophage) apoptosis through cytochrome C release.
LPCs are present as minor phospholipids in the cell membrane (≤ 3%) and in the blood plasma (8-12%).[4] Since LPCs are quickly metabolized by lysophospholipase and LPC-acyltransferase, they last only shortly in vivo. By replacing the acyl-group within the LPC with an alkyl-group, alkyl-lysophospholipids (ALP) were synthesized. These LPC analogues are metabolically stable, and several such as edelfosine, miltefosine and perifosine are under research and development as drugs against cancer and other diseases.[4][5] Lysophosphatidylcholine processing has been discovered to be an essential component of normal human brain development, those born with genes that prevent adequate uptake suffer from lethal microcephaly.[6]
LPCs occur in many foods naturally. In Starch: Chemistry and Technology third edition on page 592, the authors cite that “lysophosphatidylcholine makes up about 70% of the lipids in oat starch.” [7]
Their anti-cancer abilities are special since they do not target the cell DNA but insert into the plasma membrane and cause apoptosis through influencing several signal pathways. Therefore, their effects are independent of the proliferation state of the tumor cell.[8]
Other Names for Phosphatidylcholine in Food, Products, and Industry
Lysolecithin is a name used prior to the 1980s that was lost and now is found as lysophosphatidylcholine. In addition to several other names lysolecithin / lysophosphatidylcholine is also called hydrolyzed lecithin or hydrolysed lecithin or enzyme-modified lecithin. It is often shortened to LPC or lysoPC. The demyelinating effects of lysolecithin / lysophosphatidylcholine occur through topical application [9] and injection.[10]
Lysophosphatidylcholine could be the lost link in the Multiple Sclerosis controversy between the neurologists and the vascular surgeons since lysophosphatidylcholine has a twofold inflammation effect - lysophosphatidylcholine causes lesions on the central nervous system [11] and lysophosphatidylcholine causes vasoconstriction in the venous system.[12] More is discussed below concerning possible diseases related to excessive intake of lysophosphatidylcholine. If one has sclerosis or venous occlusion then one may want to try removing all sources of lysophosphatidylcholine as seen in these foods and products.
In the Biochemistry of Foods, Peter Eck explains recombinant DNA technologies in food. Pages 543-545 explain the use of glycerophospholipid cholesterol acyltransferase and the reaction products which are lysophospholipids. He states that “the enzyme preparation is used in egg yolk and whole eggs, in processed meats, in degumming of vegetable oils, in milk products such as cheese, and in bakery products containing eggs, such as cake products.” Then he lists each one and that in milk, the enzyme produces lysophospholipids from the phospholipids. He further explains that the enzyme preparation converts phospholipids to lysophospholipids in each of the above areas. Lysophosphatidylcholine is a lysophospholipid. This is significant and shows that there are unnaturally high amounts of lysophosphatidylcholine in enzyme-modified foods.[13]
Foods and Products with Unnaturally High Quantities of Lysophosphatidylcholine
Modified coconut oil has high levels of lysophosphatidylcholine; look at Mr. Rahman’s page 12 chart [14] to see that naturally phosphatidylcholine is 34 percent of lipids in coconut oil and lysophosphatidylcholine is only four percent. Once modified though, all that phosphatidylcholine could become hydrolyzed and be lysophosphatidylcholine – a demylenating toxin. In 2012, there was a lot said about modified coconut oil’s benefits for tooth brushing.
Here is another use for lysophosphatidylcholine – an immune activator. Vaccines.[15]
Diseases Related to Excessive Intake of Unnatural Quantities of Lysophosphatidylcholine
What effects would occur from lysophosphatidylcholine in or near the eye? Hypothetically, one suffering from macular degeneration may drop lysophosphatidylcholine, a demyelinating agent, in one's eyes. This would make the retinal vein occlude due to the vasoconstricting effects of lysophosphatidylcholine and could cause wet macular degeneration; conversely, if one is not taking a demyelinating agent into or around one's eye, then one would have dry macular degeneration and no retinal occlusion.
More research needs to be done to look into this hypothesis and as Ebrahimi and Handa said about lipid biology, “While lipoproteins in Bruch’s membrane both accumulate and can become oxidized, experimental proof of establishing how lipoprotein accumulation in Bruch’s membrane leads to RPE cell death or drusen formation is currently lacking and would establish a definitive role for lipids in AMD. In the future, it will be necessary for the epidemiologists, genetics, and molecular and cellular biologists to work together to definitively determine how lipid biology influences the development of AMD. It is hoped that greater understanding of the molecular biology of AMD lesions will provide a knowledge based on which to develop multiple treatment targets for the benefit of patients with AMD.” [16] Further study needs to be done to determine if the vasoconstriction could be reduced and the vein brought back to normal functioning by eliminating any demyelinating agents in foods, drops, or products.
In the Journal of Viral Hepatitis, Nie and Han et al. researched 99 metabolites in Acute-on-chronic liver failure and found, “Our study demonstrated the following 17 metabolites, including LysoPC(18:0), LysoPC(17:0), LysoPC(16:0), LysoPC(15:0), LysoPC(14:0), L-Threonine, Acetoacetic acid, DHAP(18:0), Phenylalanyl Phenylalanine, Bilirubin glucuronide, PA(20:4(5Z,8Z,11Z,14Z)e/2:0),m/z = 210.05, m/z = 475.172, m/z = 797.3, m/z = 599.29, m/z = 258.818 and m/z = 775.318. These 17 metabolites could be used as important potential markers for predicting the prognosis of patients with HBV-related ACLF. 11 metabolites, including LysoPC(14:0), Phenylalanyl Phenylalanine, Bilirubin glucuronide, LysoPC(22:5), m/z = 797.3, m/z = 759.286, m/z = 475.172, m/z = 599.29, m/z = 515.313, m/z = 775.318 and m/z = 809.287 showed disease-monitoring value.” This breakthrough research shows that in the acute-on-chronic liver failure the lysophosphatidylcholine and the mechanisms that directly surround it play a huge part in downfall. The other 82 metabolites studied in this paper do not have negative numbers. The LPCs are the ones implicated in liver cirrhosis and hepatitis.
Along the same lines, Neiderhiser and Maksem proved that ethanol (drinking alcohol) increases lysophosphatidylcholine absorption in the stomach.[17] Perhaps, ingesting unnaturally large amounts of lysoPC in addition to large amounts of alcohol is the cause of alcoholic liver disease.
Lysophosphatidylcholine - The Reason Why Smoking is Bad for those with Multiple Sclerosis
It is well known that smoking tobacco makes multiple sclerosis worse [18] although none can explain why, until now. Pasini and group at the University of Verona found that smoking induces phospholipase and increases lysoPC levels,[19] which makes lesions in high numbers. Pasini et al. said, “…smokers compared to non-smokers, had significantly higher levels of circulating oxLDL (44.7±9.1 versus 15.8±7.5 µg/ml respectively, P<0.001). Moreover our results showed higher plasma levels of Lp-PLA2 (256.7±63.1 versus 191.3±48.0 ng/ml respectively, P<0.001) and of lysoPC (133.8±39.4 µmol/l and 95.3±20.8 µmol/l respectively, P<0.001) in smokers compared to non-smokers. These data are presented in Table 1. We also found a significant positive correlation between plasma concentrations of ox-LDL and of LpPLA2 (r=0.654, P<0.001) and between plasma concentrations of Lp-PLA2 and of lysoPC in the whole population of subjects studied (r=0.639, P<0.001),” They went on to say, ”Moreover the correlation between lysoPC and CIMT together with the finding that lysoPC up-regulates proteoglycan synthesis suggests that lysoPC may be a link between smoking and intimal thickening.” Hypothetically, the reason not every smoker has MS may be due to a cumulative effect of lysoPC, which would depend on what foods and products everyone’s using.
See also
References
- ↑ Phosphatidylcholine and related lipids, lipidlibrary.co.uk
- ↑ Li X, Fang P, et al. (April 2016). "Mitochondrial Reactive Oxygen Species Mediate Lysophosphatidylcholine-Induced Endothelial Cell Activation". Arteriosclerosis, Thrombosis, and Vascular Biology. doi:10.1161/ATVBAHA.115.306964. PMID 27127201.
- ↑ Lauber, K; Bohn, E; Kröber, SM; Xiao, Y (2003). "Apoptotic Cells Induce Migration of Phagocytes via Caspase-3-Mediated Release of a Lipid Attraction Signal". Cell. 113 (6): 717–730. doi:10.1016/S0092-8674(03)00422-7. PMID 12809603.
- 1 2 3 Munder, PG; Modolell M; Andreesen R; Weltzien HU; Westphal O (1979). "Lysophosphatidylcholine (Lysolecithin) and its Synthetic Analogues . Immunemodulating and Other Biologic Effects". Springer Seminars in Immunopathology. 203: 187–203. doi:10.1007/bf01891668.
- ↑ Houlihan, W; Lohmeyer M; Workman P; Cheon SH (1995). "Phospholipid antitumor agents.". Medicinal Research Reviews. 15 (3): 157–223. doi:10.1002/med.2610150302. PMID 7658750.
- ↑ Guemez-Gamboa, Alicia; N Nguyen; Hongbo Yan. "Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome.". Nature Genetics. 47: 809–813. doi:10.1038/ng.3311.
- ↑ "Starch". google.com.
- ↑ van Blitterswijk, W; Verheij M (2008). "Anticancer alkylphospholipids: mechanisms of action, cellular sensitivity and resistance, and clinical prospects.". Current Pharmaceutical Design. 14 (21): 2061–74. doi:10.2174/138161208785294636. PMID 18691116.
- ↑ Griffin JW; et al. (1990). "Schwann cell proliferation following lysolecithin-induced demyelination.". J. Neurocytol. 19: 367–84. doi:10.1007/bf01188405. PMID 2391539.
- ↑ MouseDoctor. "Multiple Sclerosis Research: Research: Promoting remyelination is not a simple task". multiple-sclerosis-research.blogspot.com.
- ↑ http://www.jneurosci.org/content/18/7/2498.full.pdf
- ↑ "Kidney International - Abstract of article: Endothelial function in proteinuric renal disease". nature.com.
- ↑ "Biochemistry of Foods". google.com.
- ↑ http://fos.ubd.edu.bn/sites/default/files/2000-Paper2.pdf
- ↑ "Patent US20110135684 - Use of L-alpha-lysophosphatidylcholine to obtain the differentiation of ... - Google Patents". google.com.mx.
- ↑ http://www.hindawi.com/journals/jl/2011/802059/
- ↑ "Gastric mucosal damage induced by combination of ethanol and lysophosphatidylcholine". springer.com.
- ↑ Julie Stachowiak, Ph.D. "Smoking and Multiple Sclerosis - Smoking Mechanisms of Action in Multiple Sclerosis". About.com Health.
- ↑ "Lysophosphatidylcholine and Carotid Intima-Media Thickness in Young Smokers: A Role for Oxidized LDL-Induced Expression of PBMC Lipoprotein-Associated Phospholipase A2?". pubmedcentralcanada.ca.