Imine Diels–Alder reaction
The imine Diels-Alder reaction involves the transformation of all-carbon dienes and imine dienophiles into tetrahydropyridines.[1]
Introduction
Imines may be employed as dienophiles in hetero-Diels-Alder reactions. These reactions involve the lowest unoccupied molecular orbital (LUMO) of the imine, meaning that imines substituted with electron-withdrawing groups on nitrogen are the most reactive. The reaction may be thermal, in refluxing solvents such as benzene or others typical for Diels–Alder reactions, or acid catalyzed, again using common Diels–Alder Lewis acids such as boron trifluoride or zinc chloride. It may proceed via a concerted, [4+2] cycloaddition mechanism, although in cases of extreme polarization, addition to the imine followed by nitrogen nucleophilic attack (the "Mannich-Michael" pathway) occurs.[2] Cyclic, acyclic, and tethered imines have all been employed in the reaction with success.
(1)

Simple alkyl or aryl amines are often
generated in situ by combining an amine hydrochloride with an aldehyde.
Mechanism and stereochemistry
Prevailing mechanism
The imino Diels-Alder (IDA) reaction may occur either by a concerted or stepwise process. The lowest-energy transition state for the concerted process places the imine lone pair (or coordinated Lewis acid) in an exo position. Thus, (E) imines, in which the lone pair and larger imine carbon substituent are cis, tend to give exo products.[3]
(2)
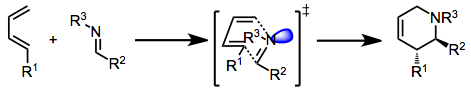
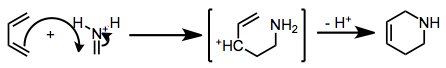
When the imine nitrogen is protonated or coordinated to a strong Lewis acid, the mechanism shifts to a stepwise, Mannich-Michael pathway.[4]
(3)
Whatever the mechanism, the transition state of cyclization is highly polarized. Thus, the regiochemistry of cycloaddition can be predicted by considering the electron-withdrawing or -donating nature of substituents on the diene. The carbon bearing the largest coefficient in the HOMO of the diene forms a bond to the imine carbon.
(4)

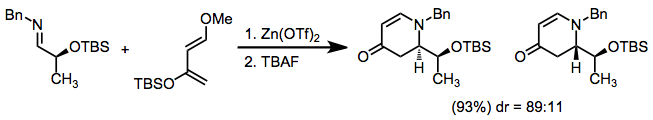
Stereoselective variants
In many cases, cyclic dienes give higher diastereoselectivities than acyclic dienes. Use of amino-acid-based chiral auxiliaries, for instance, leads to good diastereoselectivities in reactions of cyclopentadiene, but not in reactions of acyclic dienes.[5]
(6)

Chiral auxiliaries have been employed on either the imino nitrogen[6] or imino carbon[7] to effect diastereoselection.
(5)
Scope and limitations
Attaching an electron-withdrawing group to the imine nitrogen increases the reactivity of the imine. The exo isomer usually predominates (particularly when cyclic dienes are used), although selectivities vary.[8]
(7)
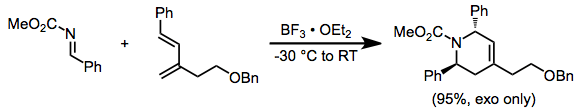
Tosylimines may be generated in situ from tosylisocyanate and aldehydes. Cycloadditions of these intermediates with dienes give single constitutional isomers, but proceed with moderate stereoselectivity.[9]
(8)

Lewis-acid catalyzed reactions of sulfonyl imines also exhibit moderate stereoselectivity.[10]
(9)

Simple unactivated imines react with hydrocarbon dienes only with the help of a Lewis acid; however, both electron-rich and electron-poor dienes react with unactivated imines when heated. Vinylketenes, for instance, afford dihydropyridones upon [4+2] cycloaddition with imines. Regio- and stereoselectivity are unusually high in reactions of this class of dienes.[11]
(10)
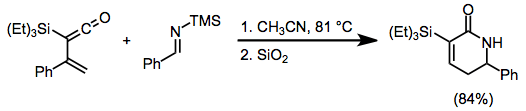
Vinylallenes react similarly in the presence of a Lewis acid, often with high diastereoselectivity.[12]
(11)

Synthetic applications
The IDA reaction has been applied to the synthesis of a number of alkaloid natural products. In this example, Danishefsky's diene is used to form a six-membered ring en route to phyllanthine.[13]
(12)

Comparison with other methods
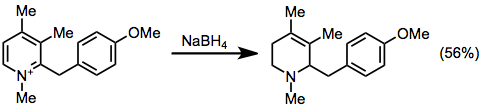
Several other methods can access the 1,2,5,6-tetrahydropyridine ring system afforded by IDA reactions. Partial reduction of pyridinium salts has been used, although regioselectivity issues arise when substituted pyridiniums are used.[14]
(13)
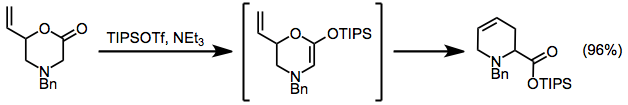
A modified Ireland-Claisen rearrangement leads to tetrahydropyridines via a silyl ketene acetal intermediate.[15]
(14)
Ring-closing olefin metathesis has also been used to establish the tetrahydropyridine ring system.[16]
(15)

References
- ↑ Heintzelman, G. R.; Meigh, I. R.; Mahajan, Y. R.; Weinreb, S. M. (2005). "Diels-Alder Reactions of Imino Dienophiles". Org. React. 65: 141. doi:10.1002/0471264180.or065.02. ISBN 0471264180.
- ↑ Waldmann, H. (1994). "Asymmetric Hetero Diels-Alder Reactions". Synthesis. 1994 (6): 535. doi:10.1055/s-1994-25519.
- ↑ Whiting, A.; Windsor, C. M. (1998). "What makes a neutral imino dieneophile undergo a thermal, non-catalysed, Diels-Alder reaction?". Tetrahedron. 54 (22): 6035. doi:10.1016/S0040-4020(98)00284-1.
- ↑ Hermitage, S.; Jay, D. A.; Whiting, A. (2002). "Evidence for the non-concerted \4+2]-cycloaddition of N-aryl imines when acting as both dienophiles and dienes under Lewis acid-catalysed conditions". Tetrahedron Lett. 43 (52): 9633. doi:10.1016/S0040-4039(02)02392-4.
- ↑ Waldmann, H. (1989). "Asymmetrische Hetero-Diels-Alder-Reaktionen in wäßriger Lösung unter Verwendung von Aminosäureestern als chiralen Auxiliaren". Liebigs Ann. Chem. 1989 (3): 231. doi:10.1002/jlac.198919890145.
- ↑ Hedberg, C.; Pinho, P.; Roth, P.; Andersson, P. G. (2000). "Diels-Alder reaction of heterocyclic imine dienophiles". J. Org. Chem. 65 (9): 2810–2. doi:10.1021/jo9916683. PMID 10808461.
- ↑ Ishimaru, K.; Watanabe, K.; Yamamoto, Y.; Akiba, K.-Y. (1994). "Stereocontrol in \4+2]Type Cycloaddition of an Aldimine Derived from (S)-Ethyl Lactate with 2-Siloxy-1,3-butadienes". Synlett. 1994 (7): 495. doi:10.1055/s-1994-22902.
- ↑ Corey, E. J.; Yuen, P.-W. (1989). "A short, stereospecific route to chiral trans-2,6-disubstituted quinuclidines". Tetrahedron Lett. 30 (43): 5825. doi:10.1016/S0040-4039(01)93481-1.
- ↑ Schrader, T.; Steglich, W. (1990). "Phosphoranaloge von Aminosäuren IV.1Synthesen ungewöhnlicher 1-Aminophosphonsäuren über Diels-Alder-Reaktionen von (N-Acyliminomethyl)phosphonsäurediethylestern". Synthesis. 1990 (12): 1153. doi:10.1055/s-1990-27122.
- ↑ Krow, G. R.; Pyun, C.; Rodebaugh, R.; Marakowski, J. (1974). "Heterodienophiles—V". Tetrahedron. 30 (17): 2977. doi:10.1016/S0040-4020(01)97542-8.
- ↑ Bennett, D. M.; Okamoto, I.; Danheiser, R. L. (1999). "Hetero 4 + 2 cycloadditions of (trialkylsilyl)vinylketenes. Synthesis of alpha,beta-unsaturated delta-valerolactones and -lactams". Org. Lett. 1 (4): 641–4. doi:10.1021/ol9907217. PMID 10823193.
- ↑ Regas, D.; Afonso, M. M.; Rodriguez, M. L.; Palenzuela, J. A. (2003). "Synthesis of octahydroquinolines through the Lewis acid catalyzed reaction of vinyl allenes and imines". J. Org. Chem. 68 (20): 7845–52. doi:10.1021/jo034480z. PMID 14510565.
- ↑ Han, G.; LaPorte, M. G.; Folmer, J. J.; Werner, K. M.; Weinreb, S. M. (2000). "Total syntheses of the Securinega alkaloids (+)-14,15-dihydronorsecurinine, (−)-norsecurinine, and phyllanthine". J. Org. Chem. 65 (20): 6293–306. doi:10.1021/jo000260z. PMID 11052071.
- ↑ Thyagarajan, G.; May, E. L. (1971). "Improved synthesis of 2-benzyl-1,2,5,6-tetrahydropyridines, precursors of analgetic 6,7-benzomorphans". J. Heterocycl. Chem. 8 (3): 465. doi:10.1002/jhet.5570080317.
- ↑ Angle, S. R.; Henry, R. M. (1998). "Studies toward the Synthesis of (+)-Palustrine: The First Asymmetric Synthesis of (−)-Methyl Palustramate". J. Org. Chem. 63 (21): 7490–7497. doi:10.1021/jo980749g. PMID 11672402.
- ↑ Deiters, A.; Martin, S. F. (2004). "Synthesis of oxygen- and nitrogen-containing heterocycles by ring-closing metathesis". Chem. Rev. 104 (5): 2199–238. doi:10.1021/cr0200872. PMID 15137789.