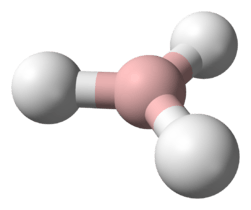
Hydrogen sulfide
 | |||
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
Hydrogen sulfide[1] | |||
| Other names
Dihydrogen monosulfide Dihydrogen sulfide | |||
| Identifiers | |||
| 7783-06-4 | |||
| 3D model (Jmol) | Interactive image | ||
| 3DMet | B01206 | ||
| 3535004 | |||
| ChEBI | CHEBI:16136 | ||
| ChEMBL | ChEMBL1200739 | ||
| ChemSpider | 391 | ||
| ECHA InfoCard | 100.029.070 | ||
| EC Number | 231-977-3 | ||
| 303 | |||
| KEGG | C00283 | ||
| MeSH | Hydrogen+sulfide | ||
| PubChem | 402 | ||
| RTECS number | MX1225000 | ||
| UNII | YY9FVM7NSN | ||
| UN number | 1053 | ||
| |||
| |||
| Properties | |||
| H2S | |||
| Molar mass | 34.08 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Rotten eggs | ||
| Density | 1.363 g dm−3 | ||
| Melting point | −82 °C (−116 °F; 191 K) | ||
| Boiling point | −60 °C (−76 °F; 213 K) | ||
| 4 g dm−3 (at 20 °C) | |||
| Vapor pressure | 1740 kPa (at 21 °C) | ||
| Acidity (pKa) | 7.0[2][3] | ||
| Basicity (pKb) | 12.9 | ||
| Refractive index (nD) |
1.000644 (0 °C)[4] | ||
| Structure | |||
| C2v | |||
| Bent | |||
| 0.97 D | |||
| Thermochemistry | |||
| 1.003 J K−1 g−1 | |||
| Std molar entropy (S |
206 J mol−1 K−1[5] | ||
| Std enthalpy of formation (ΔfH |
−21 kJ mol−1[5] | ||
| Hazards | |||
| Safety data sheet | External MSDS[6] | ||
| EU classification (DSD) |
| ||
| R-phrases | R12, R26, R50 | ||
| S-phrases | (S1/2), S9, S16, S36, S38, S45, S61 | ||
| NFPA 704 | |||
| Flash point | −82.4 °C (−116.3 °F; 190.8 K) [7] | ||
| 232 °C (450 °F; 505 K) | |||
| Explosive limits | 4.3–46% | ||
| Lethal dose or concentration (LD, LC): | |||
| LC50 (median concentration) |
713 ppm (rat, 1 hr) 673 ppm (mouse, 1 hr) 634 ppm (mouse, 1 hr) 444 ppm (rat, 4 hr)[8] | ||
| LCLo (lowest published) |
600 ppm (human, 30 min) 800 ppm (human, 5 min)[8] | ||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
C 20 ppm; 50 ppm [10-minute maximum peak][9] | ||
| REL (Recommended) |
C 10 ppm (15 mg/m3) [10-minute][9] | ||
| IDLH (Immediate danger) |
100 ppm[9] | ||
| Related compounds | |||
| Related hydrogen chalcogenides |
Water Hydrogen selenide Hydrogen telluride Hydrogen polonide Hydrogen disulfide Sulfanyl | ||
| Related compounds |
Phosphine | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Hydrogen sulfide is the chemical compound with the formula H
2S. It is a colorless gas with the characteristic foul odor of rotten eggs; it is heavier than air, very poisonous, corrosive, flammable, and explosive; properties shared with the denser hydrogen chalcogenides.
Hydrogen sulfide often results from the prokaryotic breakdown of organic matter in the absence of oxygen gas, such as in swamps and sewers; this process is commonly known as anaerobic digestion. H
2S also occurs in volcanic gases, natural gas, and in some sources of well water. It is also present in natural halite type rock salts, most notably in Himalayan Black Salt, which is mostly harvested from the mineral-rich Salt Range mountains of Pakistan. The human body produces small amounts of H
2S and uses it as a signaling molecule.
Dissolved in water, hydrogen sulfide is known as hydrosulfuric acid or sulfhydric acid, a weak acid.
Swedish chemist Carl Wilhelm Scheele is credited with having discovered hydrogen sulfide in 1777.
The British English spelling of this compound is hydrogen sulphide, but this spelling is not recommended by the International Union of Pure and Applied Chemistry or the Royal Society of Chemistry.
In 2015, hydrogen sulfide under extremely high pressure (around 150 gigapascals/1.5 million bar) was found to undergo superconducting transition near −70 °C (−94 °F), the highest temperature superconductor known to date.[10]
Properties
Hydrogen sulfide is slightly heavier than air; a mixture of H
2S and air can be explosive. Hydrogen sulfide and oxygen burn with a blue flame to form sulfur dioxide (SO
2) and water. In general, hydrogen sulfide acts as a reducing agent.
At high temperature or in the presence of catalysts, sulfur dioxide can be made to react with hydrogen sulfide to form elemental sulfur and water. This is exploited in the Claus process, the main way to convert hydrogen sulfide into elemental sulfur.
Hydrogen sulfide is slightly soluble in water and acts as a weak acid, giving the hydrosulfide ion HS− (pKa = 6.9 in 0.01–0.1 mol/litre solutions at 18 °C). A solution of hydrogen sulfide in water, known as sulfhydric acid or hydrosulfuric acid, is initially clear but over time turns cloudy. This is due to the slow reaction of hydrogen sulfide with the oxygen dissolved in water, yielding elemental sulfur, which precipitates out. The sulfide dianion S2− exists only in strongly alkaline aqueous solutions; it is exceptionally basic with a pKa > 14.
Hydrogen sulfide reacts with metal ions to form metal sulfides, which may be considered the salts of hydrogen sulfide. Some ores are sulfides. Metal sulfides often have a dark color. Lead(II) acetate paper is used to detect hydrogen sulfide because it turns grey in the presence of the gas as lead(II) sulfide is produced. Reacting metal sulfides with strong acid liberates hydrogen sulfide.
If gaseous hydrogen sulfide (a strong reducing agent) is put into contact with concentrated nitric acid (a strong oxidizer), it reacts explosively to give NOx, SOx, nitrogen, and water.
Hydrogen sulfide reacts with alcohols to form thiols, an important class of organosulfur compounds.
At pressures above 90 GPa (Gigapascal), hydrogen sulfide becomes a metallic conductor of electricity. When cooled below a critical temperature this high-pressure phase exhibits superconductivity. The critical temperature increases with pressure, ranging from 23 K at 100 GPa to 150 K at 200 GPa.[11] If hydrogen sulfide is pressurized at higher temperatures, then cooled, the critical temperature reaches 203 K (−70 °C), the highest accepted superconducting critical temperature as of 2015.[10] By substituting a small part of sulfur with phosphorus and using even higher pressures, it has been predicted that it may be possible to raise the critical temperature to above 0 °C (273 K) and achieve room-temperature superconductivity.[10]
Production
Hydrogen sulfide is most commonly obtained by its separation from sour gas, which is natural gas with high content of H
2S. It can also be produced by reacting hydrogen gas with molten elemental sulfur at about 450 °C. Hydrocarbons can replace hydrogen in this process.[12]
Sulfate-reducing (resp. sulfur-reducing) bacteria generate usable energy under low-oxygen conditions by using sulfates (resp. elemental sulfur) to oxidize organic compounds or hydrogen; this produces hydrogen sulfide as a waste product.
The standard lab preparation is to react ferrous sulfide (FeS) with a strong acid in a Kipp generator:
- FeS + 2 HCl → FeCl2 + H2S
A less well-known and more convenient alternative is to react aluminium sulfide with water:[13]
- 6 H2O + Al2S3 → 3 H2S + 2 Al(OH)3
This gas is also produced by heating sulfur with solid organic compounds and by reducing sulfurated organic compounds with hydrogen.
Hydrogen sulfide production can be costly because of the dangers involved in production.
Water heaters can aid the conversion of sulfate in water to hydrogen sulfide gas. This is due to providing a warm environment sustainable for sulfur bacteria and maintaining the reaction which interacts between sulfate in the water and the water heater anode, which is usually made from magnesium metal.[14]
Occurrence

Small amounts of hydrogen sulfide occur in crude petroleum, but natural gas can contain up to 90%. Volcanoes and some hot springs (as well as cold springs) emit some H
2S, where it probably arises via the hydrolysis of sulfide minerals, i.e. MS + H
2O → MO + H
2S. Hydrogen sulfide can be present naturally in well water, often as a result of the action of sulfate-reducing bacteria. Hydrogen sulfide is created by the human body in small doses through bacterial breakdown of proteins containing sulfur in the intestinal tract. It is also produced in the mouth and is a cause of halitosis.[15]
A portion of global H
2S emissions are due to human activity. By far the largest industrial source of H
2S is petroleum refineries: The hydrodesulfurization process liberates sulfur from petroleum by the action of hydrogen. The resulting H
2S is converted to elemental sulfur by partial combustion via the Claus process, which is a major source of elemental sulfur. Other anthropogenic sources of hydrogen sulfide include coke ovens, paper mills (using the Kraft process), tanneries and sewerage. H
2S arises from virtually anywhere where elemental sulfur comes in contact with organic material, especially at high temperatures. Depending on environmental conditions, it is responsible for deterioration of material through the action of some sulfur oxidizing microorganisms. It is called biogenic sulfide corrosion.
In 2011 it was reported that increased concentration of H
2S, possibly due to oil field practices, was observed in the Bakken formation crude and presented challenges such as "health and environmental risks, corrosion of wellbore, added expense with regard to materials handling and pipeline equipment, and additional refinement requirements".[16]
Besides living near a gas and oil drilling operations, ordinary citizens can be exposed to hydrogen sulfide by being near waste water treatment facilities, landfills and farms with manure storage. Exposure occurs through breathing contaminated air or drinking contaminated water.[17]
Uses
Production of thioorganic compounds
Several organosulfur compounds are produced using hydrogen sulfide. These include methanethiol, ethanethiol, and thioglycolic acid.
Alkali metal sulfides
Upon combining with alkali metal bases, hydrogen sulfide converts to alkali hydrosulfides such as sodium hydrosulfide and sodium sulfide, which are used in the degradation of biopolymers. The depilation of hides and the delignification of pulp by the Kraft process both are effected by alkali sulfides.
Analytical chemistry
For well over a century, hydrogen sulfide was important in analytical chemistry, in the qualitative inorganic analysis of metal ions. In these analyses, heavy metal (and nonmetal) ions (e.g., Pb(II), Cu(II), Hg(II), As(III)) are precipitated from solution upon exposure to H
2S. The components of the resulting precipitate redissolve with some selectivity.
For small-scale laboratory use in analytic chemistry, the use of thioacetamide has superseded H
2S as a source of sulfide ions.
Precursor to metal sulfides
As indicated above, many metal ions react with hydrogen sulfide to give the corresponding metal sulfides. This conversion is widely exploited. For example, gases or waters contaminated by hydrogen sulfide can be cleaned with metal sulfides. In the purification of metal ores by flotation, mineral powders are often treated with hydrogen sulfide to enhance the separation. Metal parts are sometimes passivated with hydrogen sulfide. Catalysts used in hydrodesulfurization are routinely activated with hydrogen sulfide, and the behavior of metallic catalysts used in other parts of a refinery is also modified using hydrogen sulfide.
Miscellaneous applications
Hydrogen sulfide is used to separate deuterium oxide, or heavy water, from normal water via the Girdler sulfide process.
Scientists from the University of Exeter discovered that cell exposure to small amounts of hydrogen sulfide gas can prevent mitochondrial damage. When the cell is stressed with disease, enzymes are drawn into the cell to produce small amounts of hydrogen sulfide. This study could have further implications on preventing strokes, heart disease and arthritis.[18]
Hydrogen sulfide may have anti-aging properties by blocking destructive chemicals within the cell, bearing similar properties to resveratrol, an antioxidant found in red wine.[19]
Removal from fuel gases
Hydrogen sulfide is commonly found in natural gas, biogas, and LPG. It can be removed in a number of ways.
- Reaction with iron oxide
- Gas is pumped through a container of hydrated iron(III) oxide, which combines with hydrogen sulfide.
- Fe
2O
3(s) + H
2O(l) + 3 H
2S(g) → Fe
2S
3(s) + 4 H
2O(l)
- Fe
- In order to regenerate iron(III) oxide, the container must be taken out of service, flooded with water and aerated.
- 2 Fe
2S
3(s) + 3 O
2(g) + 2 H
2O(l) → 2 Fe
2O
3(s) + 2 H
2O(l) + 6 S(s)
- 2 Fe
- On completion of the regeneration reaction the container is drained of water and can be returned to service. The advantage of this system is that it is completely passive during the extraction phase.[20]
- Gas is pumped through a container of hydrated iron(III) oxide, which combines with hydrogen sulfide.
- Hydrodesulfurization
- Hydrodesulfurization is a more complex method of removing sulfur from fuels.
- Filtration through activated carbon
- A gas stream containing hydrogen sulfide can be purified by passing it through a suitably designed filter containing activated carbon. This method is typically used for odor abatement at municipal sewage works and for the purification of landfill biogas, prior to its use in combined heat and power (CHP) engines, or injection into the gas grid. Filters containing activated carbon are also used in the offshore industry.
- Treatment by plasma
- Plasma dissociation of hydrogen sulfide is a new method of treatment which utilizes plasma to dissociate hydrogen sulfide into hydrogen gas and elemental sulfur.
Removal from water
Hydrogen sulfide can be removed effectively from drinking water and there are a number of processes designed for this purpose. However, the preferred method can change according to the level of concentration in water. Drinking water should be checked for hydrogen sulfide levels, especially if using ground water due to low dissolved oxygen levels.[21]
- Continuous chlorination
- For levels up to 75 mg/L chlorine is used in the purification process as an oxidizing chemical to react with hydrogen sulfide. This reaction yields insoluble solid sulfur. Usually the chlorine used is in the form of sodium hypochlorite.[22]
- Aeration
- Nitrate addition
- Calcium nitrate can be used to prevent hydrogen sulfide formation in wastewater streams.
Safety
Hydrogen sulfide is a highly toxic and flammable gas (flammable range: 4.3–46%). Being heavier than air, it tends to accumulate at the bottom of poorly ventilated spaces. Although very pungent at first, it quickly deadens the sense of smell, so victims may be unaware of its presence until it is too late. For safe handling procedures, a hydrogen sulfide material safety data sheet (MSDS) should be consulted.[24]
Toxicity
Hydrogen sulfide is considered a broad-spectrum poison, meaning that it can poison several different systems in the body, although the nervous system is most affected. The toxicity of H
2S is comparable with that of carbon monoxide.[25] It forms a complex bond with iron in the mitochondrial cytochrome enzymes, thus preventing cellular respiration.
Since hydrogen sulfide occurs naturally in the body, the environment and the gut, enzymes exist in the body capable of detoxifying it by oxidation to (harmless) sulfate.[26] Hence, low levels of hydrogen sulfide may be tolerated indefinitely.
At some threshold level, believed to average around 300–350 ppm, the oxidative enzymes become overwhelmed. Many personal safety gas detectors, such as those used by utility, sewage and petrochemical workers, are set to alarm at as low as 5 to 10 ppm and to go into high alarm at 15 ppm.
A diagnostic clue of extreme poisoning by H
2S is the discolouration of copper coins in the pockets of the victim. Treatment involves immediate inhalation of amyl nitrite, injections of sodium nitrite or administration of 4-dimethylaminophenol in combination with inhalation of pure oxygen, administration of bronchodilators to overcome eventual bronchospasm, and in some cases hyperbaric oxygen therapy (HBOT).[25] HBOT has clinical and anecdotal support.[27][28][29]
Exposure to lower concentrations can result in eye irritation, a sore throat and cough, nausea, shortness of breath, and fluid in the lungs (pulmonary edema).[25] These effects are believed to be due to the fact that hydrogen sulfide combines with alkali present in moist surface tissues to form sodium sulfide, a caustic.[30] These symptoms usually go away in a few weeks.
Long-term, low-level exposure may result in fatigue, loss of appetite, headaches, irritability, poor memory, and dizziness. Chronic exposure to low level H
2S (around 2 ppm) has been implicated in increased miscarriage and reproductive health issues among Russian and Finnish wood pulp workers,[31] but the reports have not (as of circa 1995) been replicated.
Short-term, high-level exposure can induce immediate collapse, with loss of breathing and a high probability of death. If death does not occur, high exposure to hydrogen sulfide can lead to cortical pseudolaminar necrosis, degeneration of the basal ganglia and cerebral edema.[25] Although respiratory paralysis may be immediate, it can also be delayed up to 72 hours.[32]
- 0.00047 ppm or 0.47 ppb is the odor threshold, the point at which 50% of a human panel can detect the presence of an odor without being able to identify it.[33]
- 10 ppm is the OSHA permissible exposure limit (PEL) (8 hour time-weighted average).[15]
- 10–20 ppm is the borderline concentration for eye irritation.
- 20 ppm is the acceptable ceiling concentration established by OSHA.[15]
- 50 ppm is the acceptable maximum peak above the ceiling concentration for an 8-hour shift, with a maximum duration of 10 minutes.[15]
- 50–100 ppm leads to eye damage.
- At 100–150 ppm the olfactory nerve is paralyzed after a few inhalations, and the sense of smell disappears, often together with awareness of danger.[34][35]
- 320–530 ppm leads to pulmonary edema with the possibility of death.[25]
- 530–1000 ppm causes strong stimulation of the central nervous system and rapid breathing, leading to loss of breathing.
- 800 ppm is the lethal concentration for 50% of humans for 5 minutes' exposure (LC50).
- Concentrations over 1000 ppm cause immediate collapse with loss of breathing, even after inhalation of a single breath.
Incidents
Hydrogen sulfide was used by the British Army as a chemical weapon during World War I. It was not considered to be an ideal war gas, but, while other gases were in short supply, it was used on two occasions in 1916.[36]
In 1975, a hydrogen sulfide release from an oil drilling operation in Denver City, Texas, killed nine people and caused the state legislature to focus on the deadly hazards of the gas. State Representative E L Short took the lead in endorsing an investigation by the Texas Railroad Commission and urged that residents be warned "by knocking on doors if necessary" of the imminent danger stemming from the gas. One may die from the second inhalation of the gas, and a warning itself may be too late.[37]
A dump of toxic waste containing hydrogen sulfide is believed to have caused 17 deaths and thousands of illnesses in Abidjan, on the West African coast, in the 2006 Côte d'Ivoire toxic waste dump.
In 2014, Levels of Hydrogen Sulfide as high as 83 ppm have been detected at a recently built mall in Thailand called Siam Square One at the Siam Square area. Shop tenants at the mall reported health complications such as sinus inflammation, breathing difficulties and eye irritation. After investigation it was determined that the large amount of gas originated from imperfect treatment and disposal of waste water in the building.[38]
In November 2014, a substantial amount of hydrogen sulfide gas shrouded the central, eastern and southeastern parts of Moscow. Residents living in the area were urged to stay indoors by the emergencies ministry. Although the exact source of the gas was not known, blame had been placed on a Moscow oil refinery.[39]
In June 2016, a mother and her daughter were found deceased in their Porsche SUV. The medical examiner determined the cause to be hydrogen sulfide intoxication from the vehicles battery located under the driver seat.[40]
Suicides
The gas, produced by mixing certain household ingredients, was used in a suicide wave in 2008 in Japan.[41] The wave prompted staff at Tokyo's suicide prevention center to set up a special hot line during "Golden Week", as they received an increase in calls from people wanting to kill themselves during the annual May holiday.[42]
As of 2010, this phenomenon has occurred in a number of US cities, prompting warnings to those arriving at the site of the suicide.[43][44][45][46][47] These first responders, such as emergency services workers or family members are at risk of death from inhaling lethal quantities of the gas, or by fire.[48][49] Local governments have also initiated campaigns to prevent such suicides.
Function in the body
Hydrogen sulfide is produced in small amounts by some cells of the mammalian body and has a number of biological signaling functions. (Only two other such gases are currently known: nitric oxide (NO) and carbon monoxide (CO).)
The gas is produced from cysteine by the enzymes cystathionine beta-synthase, cystathionine gamma-lyase and 3-mercaptopyruvate sulfurtransferase. Hydrogen sulfide acts as an endothelium-derived relaxing factor (EDRF) and as an endothelium-derived hyperpolarizing factor (EDHF).[50] It acts as a relaxant of smooth muscle and as a vasodilator[51] and is also active in the brain, where it increases the response of the NMDA receptor and facilitates long term potentiation,[52] which is involved in the formation of memory.
Eventually the gas is converted to sulfite in the mitochondria by thiosulfate reductase, and the sulfite is further oxidized to thiosulfate and sulfate by sulfite oxidase. The sulfates are excreted in the urine.[53]
Due to its effects similar to nitric oxide (without its potential to form peroxides by interacting with superoxide), hydrogen sulfide is now recognized as potentially protecting against cardiovascular disease.[51] The cardioprotective role effect of garlic is caused by catabolism of the polysulfide group in allicin to H
2S, a reaction that could depend on reduction mediated by glutathione.[54]
Though both nitric oxide (NO) and hydrogen sulfide have been shown to relax blood vessels, their mechanisms of action are different: while NO activates the enzyme guanylyl cyclase, H
2S activates ATP-sensitive potassium channels in smooth muscle cells. Researchers are not clear how the vessel-relaxing responsibilities are shared between nitric oxide and hydrogen sulfide. However, there exists some evidence to suggest that nitric oxide does most of the vessel-relaxing work in large vessels and hydrogen sulfide is responsible for similar action in smaller blood vessels.[55]
Recent findings suggest strong cellular crosstalk of NO and H
2S,[56] demonstrating that the vasodilatatory effects of these two gases are mutually dependent. Additionally, H
2S reacts with intracellular S-nitrosothiols to form the smallest S-nitrosothiol (HSNO), and a role of hydrogen sulfide in controlling the intracellular 'S-nitrosothiol pool has been suggested.[57]
Like nitric oxide, hydrogen sulfide is involved in the relaxation of smooth muscle that causes erection of the penis, presenting possible new therapy opportunities for erectile dysfunction.[58][59]
Hydrogen sulfide, similar to carbon monoxide (see carbon monoxide#Normal human physiology) and nitric oxide (see nitric oxide#Biological functions), possesses Specialized pro-resolving mediators activity. That is, it blunts, reverses, and promotes the healing of diverse inflammatory reactions. In whole animal, animal tissue, and human tissue studies, hydrogen sulfide: a) suppresses the expression of ICAM-1 and P-selectin adhesion molecules on vascular endothelial cells and LFA-1 adhesion molecules on leukocytes thereby inhibiting pro-inflammatory leukocytes from moving out of the circulation into tissue sites of inflammation; b) acts as a scavenger to neutralizes toxic substances (e.g. superoxide anion, peroxynitrite, hypochlorous acid, and hydrogen peroxide) released by leukocytes in inflamed tissues; c) renders pro-inflammatory tissue macrophages hypo-responsive to inflammatory stimuli; d) inhibits inflammatory cells from expressing pro-inflammatory cytokines such as TNFα, Interleukin 2, Interleukin 23 while stimulating their expression of the anti-inflammatory cytokine, Interleukin 10; and e) stimulates leukocyte apoptosis thereby promoting the removal of these potentially toxic cells from inflamed tissues. In animal models, hydrogen sulfide also promotes, and appears to be a natural mediator responsible for, repairing damaged tissues such as those due to Hypoxia and stomach ulcers.[60]
Involvement in diseases
Hydrogen sulfide deficiency after heart attack
A hydrogen sulfide (H2S) deficiency can be detrimental to the vascular function after an acute myocardial infarction (AMI).[61] AMIs can lead to cardiac dysfunction through two distinct changes; increased oxidative stress via free radical accumulation and decreased NO bioavailability.[62] Free radical accumulation occurs due to increased electron transport uncoupling at the active site of endothelial nitric oxide synthase (eNOS), an enzyme involved in converting L-arginine to NO.[61][62] During an AMI, oxidative degradation of tetrahydrobiopterin (BH4), a cofactor in NO production, limits BH4 availability and limits NO productionby eNOS.[62] Instead, eNOS reacts with oxygen, another cosubstrates involved in NO production. The products of eNOS are reduced to superoxides, increasing free radical production and oxidative stress within the cells.[61] A H2S deficiency impairs eNOS activity by limiting Akt activation and inhibiting Akt phosphorylation of the eNOSS1177 activation site.[61][63] Instead, Akt activity is increased to phosphorylate the eNOST495 inhibition site, downregulating eNOS production of NO.[61][63]
H2S therapy uses a H2S donor, such as diallyl trisulfide (from garlic), to increase the supply of H2S to an AMI patient. H2S donors reduce myocardial injury and reperfusion complications.[61] Increased H2S levels within the body will react with oxygen to produce sulfane sulfur, a storage intermediate for H2S.[61] H2S pools in the body attracts oxygen to react with excess H2S and eNOS to increase NO production.[61] With increased use of oxygen to produce more NO, less oxygen is available to react with eNOS to produce superoxides during an AMI, ultimately lowering the accumulation of reactive oxygen species (ROS).[61] Furthermore, decreased accumulation of ROS lowers oxidative stress in vascular smooth muscle cells, decreasing oxidative degeneration of BH4.[62] Increased BH4 cofactor contributes to increased production of NO within the body.[62] Higher concentrations of H2S directly increase eNOS activity through Akt activation to increase phosphorylation of the eNOSS1177 activation site, and decrease phosphorylation of the eNOST495 inhibition site.[61][63] This phosphorylation process upregulates eNOS activity, catalyzing more conversion of L-arginine to NO.[61][63] Increased NO production enables soluble guanylyl cyclase (sGC) activity, leading to an increased conversion of guanosine triphosphate (GTP) to 3′,5′-cyclic guanosine monophosphate (cGMP).[64] In H2S therapy immediately following an AMI, increased cGMP triggers an increase in protein kinase G (PKG) activity.[65] PKG reduces intracellular Ca2+ in vascular smooth muscle to increase smooth muscle relaxation and promote blood flow.[65] PKG also limits smooth muscle cell proliferation, reducing intima thickening following AMI injury, ultimately decreasing myocardial infarct size.[61][64]
Other diseases
In Alzheimer's disease the brain's hydrogen sulfide concentration is severely decreased.[66] In a certain rat model of Parkinson's disease, the brain's hydrogen sulfide concentration was found to be reduced, and administering hydrogen sulfide alleviated the condition.[67] In trisomy 21 (Down syndrome) the body produces an excess of hydrogen sulfide.[53] Hydrogen sulfide is also involved in the disease process of type 1 diabetes. The beta cells of the pancreas in type 1 diabetes produce an excess of the gas, leading to the death of these cells and to a reduced production of insulin by those that remain.[55]
In animal disease models caused and/or promoted by pathological inflammation, e.g. chronic inflammatory diseases (see Inflammation), drugs causing the release of hydrogen sulfide (e.g. ATB-429 and hydrogen sulfide-releasing NSAID) have shown clinically significant effects and are in development for use in humans.[60][68]
Induced hypothermia and suspended animation
In 2005, it was shown that mice can be put into a state of suspended animation-like hypothermia by applying a low dosage of hydrogen sulfide (81 ppm H
2S) in the air. The breathing rate of the animals sank from 120 to 10 breaths per minute and their temperature fell from 37 °C to just 2 °C above ambient temperature (in effect, they had become cold-blooded). The mice survived this procedure for 6 hours and afterwards showed no negative health consequences.[69] In 2006 it was shown that the blood pressure of mice treated in this fashion with hydrogen sulfide did not significantly decrease.[70]
A similar process known as hibernation occurs naturally in many mammals and also in toads, but not in mice. (Mice can fall into a state called clinical torpor when food shortage occurs.) If the H
2S-induced hibernation can be made to work in humans, it could be useful in the emergency management of severely injured patients, and in the conservation of donated organs. In 2008, hypothermia induced by hydrogen sulfide for 48 hours was shown to reduce the extent of brain damage caused by experimental stroke in rats.[71]
As mentioned above, hydrogen sulfide binds to cytochrome oxidase and thereby prevents oxygen from binding, which leads to the dramatic slowdown of metabolism. Animals and humans naturally produce some hydrogen sulfide in their body; researchers have proposed that the gas is used to regulate metabolic activity and body temperature, which would explain the above findings.[72]
Two recent studies cast doubt that the effect can be achieved in larger mammals. A 2008 study failed to reproduce the effect in pigs, concluding that the effects seen in mice were not present in larger mammals.[73] Likewise a paper by Haouzi et al. noted that there is no induction of hypometabolism in sheep, either.[74]
At the February 2010 TED conference, Mark Roth announced that hydrogen sulfide induced hypothermia in humans had completed Phase I clinical trials.[75] The clinical trials commissioned by the company he helped found, Ikaria, were however withdrawn or terminated by August 2011.[76][77]
Participant in the sulfur cycle

Hydrogen sulfide is a central participant in the sulfur cycle, the biogeochemical cycle of sulfur on Earth.[78]
In the absence of oxygen, sulfur-reducing and sulfate-reducing bacteria derive energy from oxidizing hydrogen or organic molecules by reducing elemental sulfur or sulfate to hydrogen sulfide. Other bacteria liberate hydrogen sulfide from sulfur-containing amino acids; this gives rise to the odor of rotten eggs and contributes to the odor of flatulence.
As organic matter decays under low-oxygen (or hypoxic) conditions (such as in swamps, eutrophic lakes or dead zones of oceans), sulfate-reducing bacteria will use the sulfates present in the water to oxidize the organic matter, producing hydrogen sulfide as waste. Some of the hydrogen sulfide will react with metal ions in the water to produce metal sulfides, which are not water-soluble. These metal sulfides, such as ferrous sulfide FeS, are often black or brown, leading to the dark color of sludge.
Several groups of bacteria can use hydrogen sulfide as fuel, oxidizing it to elemental sulfur or to sulfate by using dissolved oxygen, metal oxides (e.g., Fe oxyhydroxides and Mn oxides) or nitrate as oxidant.[79]
The purple sulfur bacteria and the green sulfur bacteria use hydrogen sulfide as electron donor in photosynthesis, thereby producing elemental sulfur. (In fact, this mode of photosynthesis is older than the mode of cyanobacteria, algae, and plants, which uses water as electron donor and liberates oxygen.)
The biochemistry of hydrogen sulfide is an important part of the chemistry of the iron-sulfur world. In this model of the origin of life on Earth, geologically produced hydrogen sulfide is postulated as an electron donor driving the reduction of carbon dioxide.[80]
Mass extinctions

Hydrogen sulfide has been implicated in several mass extinctions that have occurred in the Earth's past. In particular, a buildup of hydrogen sulfide in the atmosphere may have caused the Permian-Triassic extinction event 252 million years ago.[81]
Organic residues from these extinction boundaries indicate that the oceans were anoxic (oxygen-depleted) and had species of shallow plankton that metabolized H
2S. The formation of H
2S may have been initiated by massive volcanic eruptions, which emitted carbon dioxide and methane into the atmosphere, which warmed the oceans, lowering their capacity to absorb oxygen that would otherwise oxidize H
2S. The increased levels of hydrogen sulfide could have killed oxygen-generating plants as well as depleted the ozone layer, causing further stress. Small H
2S blooms have been detected in modern times in the Dead Sea and in the Atlantic ocean off the coast of Namibia.[81]
Life adapted to hydrogen sulfide
High levels of hydrogen sulfide are lethal to most animals, but a few highly specialized species (extremophiles) do thrive in habitats that are rich in this chemical.[82]
Freshwater springs rich in hydrogen sulfide are mainly home to invertebrates, but also include a small number of fish: Cyprinodon bobmilleri (a pupfish from Mexico), Limia sulphurophila (a poeciliid from the Dominican Republic), Gambusia eurystoma (a poeciliid from Mexico), and a few Poecilia (poeciliids from Mexico).[82][83]
In the deep sea, hydrothermal vents and cold seeps with high levels of hydrogen sulfide are home to a number of extremely specialized lifeforms, ranging from bacteria to fish.[84] Because of the absence of light at these depths, these ecosystems rely on chemosynthesis rather than photosynthesis.[85] Movile Cave life forms are also adapted to high levels of the gas.
See also
References
- ↑ "Hydrogen Sulfide - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- ↑ Perrin, D.D. (1982). Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution (2nd ed.). Oxford: Pergamon Press.
- ↑ Bruckenstein, S.; Kolthoff, I.M., in Kolthoff, I.M.; Elving, P.J. Treatise on Analytical Chemistry, Vol. 1, pt. 1; Wiley, NY, 1959, pp. 432–433.
- ↑ Patnaik, Pradyot (2002). Handbook of Inorganic Chemicals. McGraw-Hill. ISBN 0-07-049439-8.
- 1 2 Zumdahl, Steven S. (2009). Chemical Principles (6th ed.). Houghton Mifflin Company. p. A23. ISBN 0-618-94690-X.
- ↑ http://msds.chem.ox.ac.uk/HY/hydrogen_sulfide.html
- ↑ "Hydrogen sulfide". npi.gov.au.
- 1 2 "Hydrogen sulfide". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 "NIOSH Pocket Guide to Chemical Hazards #0337". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 Cartlidge, Edwin (18 August 2015). "Superconductivity record sparks wave of follow-up physics". Nature News. Retrieved 18 August 2015.
- ↑ Drozdov, A.; Eremets, M. I.; Troyan, I. A. (2014). "Conventional superconductivity at 190 K at high pressures". arXiv:1412.0460
 [cond-mat.supr-con].
[cond-mat.supr-con]. - ↑ Tournier-Lasserve, Jacques. "Hydrogen Sulfide". Ullmann's Encyclopedia of Chemical Industry.
- ↑ McPherson, William (1913). Laboratory manual. Boston: Ginn and Company. p. 445.
- ↑ "Why Does My Water Smell Like Rotten Eggs? Hydrogen Sulfide and Sulfur Bacteria in Well Water". Minnesota Department of Health. Minnesota Department of Health. Retrieved 1 December 2014.
- 1 2 3 4 Agency for Toxic Substances and Disease Registry (July 2006). "Toxicological Profile For Hydrogen Sulfide" (PDF). p. 154. Retrieved 2012-06-20.
- ↑ OnePetro. "Home - OnePetro". onepetro.org.
- ↑ "Hydrogen Sulfide" (PDF). Agency for Toxic Substances and Disease Registry.
- ↑ Stampler, Laura. "A Stinky Compound May Protect Against Cell Damage, Study Finds". Time. Time. Retrieved 1 December 2014.
- ↑ Khan, Natasha. "Rotten Egg Gas Seen Offering Promise of Extending Life". Bloomberg. Bloomberg. Retrieved 1 December 2014.
- ↑ "Marcab Co Inc". Marcab Co Inc. Retrieved 2013-12-19.
- ↑ Lemley, Ann T.; Schwartz, John J.; Wagenet, Linda P. "Hydrogen Sulfide in Household Drinking Water" (PDF). Cornell University.
- ↑ "Hydrogen Sulfide (Rotten Egg Odor) in Pennsylvania Groundwater Wells". Penn State. Penn State College of Agricultural Sciences. Retrieved 1 December 2014.
- ↑ McFarland, Mark L.; Provin, T. L. "Hydrogen Sulfide in Drinking Water Treatment Causes and Alternatives" (PDF). Texas A&M University. Retrieved 1 December 2014.
- ↑ Iowa State University, Department of Chemistry MSDS. "Hydrogen Sulfide Material Safety Data Sheet" (PDF). Retrieved 2009-03-14.
- 1 2 3 4 5 Lindenmann, J.; Matzi, V.; Neuboeck, N.; Ratzenhofer-Komenda, B.; Maier, A; Smolle-Juettner, F. M. (December 2010). "Severe hydrogen sulphide poisoning treated with 4-dimethylaminophenol and hyperbaric oxygen". Diving and Hyperbaric Medicine. 40 (4): 213–217. PMID 23111938. Retrieved 2013-06-07.
- ↑ Ramasamy, S.; Singh, S.; Taniere, P.; Langman, M. J. S.; Eggo, M. C. (2006). "Sulfide-detoxifying enzymes in the human colon are decreased in cancer and upregulated in differentiation". Am. J. Physiol. Gastrointest. Liver Physiol. 291 (2): G288–G296. doi:10.1152/ajpgi.00324.2005. PMID 16500920. Retrieved 2007-10-20.
- ↑ Gerasimon, G.; Bennett, S.; Musser, J.; Rinard, J. (May 2007). "Acute hydrogen sulfide poisoning in a dairy farmer". Clin. Toxicol. (Phila.). 45 (4): 420–423. doi:10.1080/15563650601118010. PMID 17486486. Retrieved 2008-07-22.
- ↑ Belley, R.; Bernard, N.; Côté, M; Paquet, F.; Poitras, J. (July 2005). "Hyperbaric oxygen therapy in the management of two cases of hydrogen sulfide toxicity from liquid manure". CJEM. 7 (4): 257–261. doi:10.1017/s1481803500014408. PMID 17355683. Retrieved 2008-07-22.
- ↑ Hsu, P.; Li, H.-W.; Lin, Y.-T. (1987). "Acute hydrogen sulfide poisoning treated with hyperbaric oxygen". J. Hyperbaric Med. 2 (4): 215–221. ISSN 0884-1225. Retrieved 2008-07-22.
- ↑ Lewis, R.J. (1996). Sax's Dangerous Properties of Industrial Materials. 1–3 (9th ed.). New York, NY: Van Nostrand Reinhold.
- ↑ Hemminki, K.; Niemi, M. L. (1982). "Community study of spontaneous abortions: relation to occupation and air pollution by sulfur dioxide, hydrogen sulfide, and carbon disulfide". Int. Arch. Occup. Environ. Health. 51 (1): 55–63. doi:10.1007/bf00378410. PMID 7152702.
- ↑ "The chemical suicide phenomenon". Firerescue1.com. 2011-02-07. Retrieved 2013-12-19.
- ↑ Iowa State University Extension (May 2004). "The Science of Smell Part 1: Odor perception and physiological response" (PDF). PM 1963a. Retrieved 2012-06-20.
- ↑ USEPA; Health and Environmental Effects Profile for Hydrogen Sulfide p.118-8 (1980) ECAO-CIN-026A
- ↑ Zenz, C.; Dickerson, O.B.; Horvath, E.P. (1994). Occupational Medicine. (3rd ed.). St. Louis, MO. p. 886.
- ↑ Foulkes, Charles Howard (2001) [First published Blackwood & Sons, 1934]. "Gas!" The story of the special brigade. Published by Naval & Military P. p. 105. ISBN 1-84342-088-0.
- ↑ Howard Swindle, "The Deadly Smell of Success". Texas Monthly, June 1975. June 1975. Retrieved December 14, 2010.
- ↑ "Do not breathe: Dangerous, toxic gas found at Siam Square One". Coconuts Bangkok. Coconuts Media. Retrieved 20 November 2014.
- ↑ "Russian capital Moscow shrouded in noxious gas". BBC News Europe. British Broadcasting Corporation. Retrieved 1 December 2014.
- ↑ Kealing, Bob. "Medical examiner confirms suspected cause of deaths in Turnpike mystery". Retrieved 2016-10-04.
- ↑ "Dangerous Japanese 'Detergent Suicide' Technique Creeps Into U.S". Wired.com. Wired (magazine). March 13, 2009.
- ↑ Namiki, Noriko (2008-05-22). "Terrible Twist in Japan Suicide Spates - ABC News". Abcnews.go.com. Retrieved 2013-12-19.
- ↑ http://info.publicintelligence.net/LARTTAChydrogensulfide.pdf
- ↑ http://info.publicintelligence.net/MAchemicalsuicide.pdf
- ↑ http://info.publicintelligence.net/illinoisH2Ssuicide.pdf
- ↑ http://info.publicintelligence.net/NYhydrogensulfide.pdf
- ↑ http://info.publicintelligence.net/KCTEWhydrogensulfide.pdf
- ↑ dhmh.maryland.gov Archived January 3, 2012, at the Wayback Machine.
- ↑ Scoville, Dean (April 2011). "Chemical Suicides - Article - POLICE Magazine". Policemag.com. Retrieved 2013-12-19.
- ↑ Paul, B. D.; Snyder, S. H. "H2S signalling through protein sulfhydration and beyond.". nih.gov.
- 1 2 Lefer, David J. (November 2007). "A new gaseous signaling molecule emerges: Cardioprotective role of hydrogen sulfide". PNAS. 104 (46): 17907–17908. Bibcode:2007PNAS..10417907L. doi:10.1073/pnas.0709010104. PMC 2084269
 . PMID 17991773. Retrieved 2008-09-26.
. PMID 17991773. Retrieved 2008-09-26. - ↑ Kimura, Hideo (2002). "Hydrogen sulfide as a neuromodulator". Molecular Neurobiology. 26 (1): 13–19. doi:10.1385/MN:26:1:013. PMID 12392053.
- 1 2 Kamoun, Pierre (July 2004). "H2S, a new neuromodulator". Médecine/Sciences. 20 (6–7): 697–700. doi:10.1051/medsci/2004206-7697. PMID 15329822.
- ↑ Benavides, Gloria A; Squadrito, Giuseppe L; Mills, Robert W; Patel, Hetal D; Isbell, T Scott; Patel, Rakesh P; Darley-Usmar, Victor M; Doeller, Jeannette E; Kraus, David W (2007-11-13). "Hydrogen sulfide mediates the vasoactivity of garlic". Proceedings of the National Academy of Sciences of the United States of America. 104 (46): 17977–17982. Bibcode:2007PNAS..10417977B. doi:10.1073/pnas.0705710104. PMC 2084282
 . PMID 17951430.
. PMID 17951430. - 1 2 Toxic Gas, Lifesaver, Scientific American, March 2010
- ↑ Coletta, C.; Papapetropoulos, A.; Erdelyi, K.; Olah, G.; Modis, K.; Panopoulos, P.; Asimakopoulou, A.; Gero, D.; Sharina, I.; Martin, E.; Szabo, C. (2012). "Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation". Proceedings of the National Academy of Sciences. 109 (23): 9161–9166. doi:10.1073/pnas.1202916109. PMC 3384190
 . PMID 22570497.
. PMID 22570497. - ↑ Filipovic, M. R.; Miljkovic, J. L.; Nauser, T.; Royzen, M.; Klos, K.; Shubina, T.; Koppenol, W. H.; Lippard, S. J.; Ivanović-Burmazović, I. (2012). "Chemical Characterization of the Smallest S-Nitrosothiol, HSNO; Cellular Cross-talk of H2S and S-Nitrosothiols". Journal of the American Chemical Society. 134 (29): 12016–12027. doi:10.1021/ja3009693. PMC 3408084
 . PMID 22741609.
. PMID 22741609. - ↑ D'Emmanuele di Villa Bianca, Roberta; Sorrentino, Raffaella; Maffia, Pasquale; Mirone, Vincenzo; Imbimbo, Ciro; Fusco, Ferdinando; De Palma, Raffaele; Ignarro, Louis J.; Cirino, Giuseppe (2009). "Hydrogen sulfide as a mediator of human corpus cavernosum smooth-muscle relaxation". PNAS. 106 (11): 4513–4518. Bibcode:2009PNAS..106.4513D. doi:10.1073/pnas.0807974105. PMC 2657379
 . PMID 19255435.
. PMID 19255435. - ↑ "Hydrogen Sulfide: Potential Help for ED". WebMD. March 2, 2009.
- 1 2 Wallace JL, Ianaro A, Flannigan KL, Cirino G (2015). "Gaseous mediators in resolution of inflammation". Seminars in Immunology. 27 (3): 227–33. doi:10.1016/j.smim.2015.05.004. PMID 26095908.
- 1 2 3 4 5 6 7 8 9 10 11 12 King, A.; Polhemus, D. J.; Bhushan, S.; Otsuka, H.; Kondo, K.; Nicholson, C. K.; Bradley, J. M.; Islam, K. N.; Calvert, J. W.; Tao, Y.-X.; Dugas, T. R.; Kelley, E. E.; Elrod, J. W.; Huang, P. L.; Wang, R.; Lefer, D. J. (January 2014). "Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent". PNAS. 111 (Early Edition): 1–6. Bibcode:2014PNAS..111.3182K. doi:10.1073/pnas.1321871111.
- 1 2 3 4 5 Alp, N. J.; Channon, K. M. (2003). "Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease". Journal of the American Heart Association. 24: 413–420. doi:10.1161/01.ATV0000110785.96039.f6 (inactive 2015-02-01).
- 1 2 3 4 Coletta, Ciro; Papapetropoulos, Andreas; Erdelyi, K.; Olah, G.; Modis, K.; Panopoulos, P.; Asimakopoulou, A.; Gero, D.; Sharina, I.; Martin, E.; Szabo, C. (April 2012). "Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation". PNAS. 109 (23): 9161–9166. Bibcode:2012PNAS..109.9161C. doi:10.1073/pnas.1202916109. PMC 3384190
 . PMID 22570497.
. PMID 22570497. - 1 2 Boerth, N. J.; Dey, N. B.; Cornwell, T. L.; Lincoln, T. M. (1997). "Cyclic GMP-dependent protein kinase regulates vascular smooth muscle cell phenotype". Journal of Vascular Research. 34 (4): 245–259. doi:10.1159/000159231. PMID 9256084.
- 1 2 Lincoln, T. M.; Cornwell, T. L.; Taylor, A. E. (March 1990). "cGMP-dependent protein kinase mediates the reduction of Ca2+ by cAMP in vascular smooth muscle cells". American Journal of Physiology. 258 (3): C399–C407. PMID 2156436.
- ↑ Eto, Ko; Asada, Takashi; Arima, Kunimasa; Makifuchi, Takao; Kimura, Hideo (2002-05-24). "Brain hydrogen sulfide is severely decreased in Alzheimer's disease". Biochemical and Biophysical Research Communications. 293 (5): 1485–1488. doi:10.1016/S0006-291X(02)00422-9. PMID 12054683.
- ↑ Hu, L. F.; Lu, M.; Tiong, C. X.; Dawe, G. S.; Hu, G.; Bian, J. S. (2010). "Neuroprotective effects of hydrogen sulfide on Parkinson's disease rat models". Aging Cell. 9 (2): 135–146. doi:10.1111/j.1474-9726.2009.00543.x. PMID 20041858.
- ↑ Gemici B, Elsheikh W, Feitosa KB, Costa SK, Muscara MN, Wallace JL (2015). "H2S-releasing drugs: anti-inflammatory, cytoprotective and chemopreventative potential". Nitric Oxide : Biology and Chemistry / Official Journal of the Nitric Oxide Society. 46: 25–31. doi:10.1016/j.niox.2014.11.010. PMID 25461269.
- ↑ "BBC News - Science/Nature - Mice put in 'suspended animation'". bbc.co.uk.
- ↑ "BBC News - Science/Nature - Mice put in 'suspended animation'". bbc.co.uk.
- ↑ Florian, B.; Vintilescu, R.; Balseanu, A. T.; Buga, A.-M.; Grisk, O.; Walker, L. C.; Kessler, C.; Popa-Wagner, A. (2008). "Long-term hypothermia reduces infarct volume in aged rats after focal ischemia". Neuroscience Letters. 438 (2): 180–185. doi:10.1016/j.neulet.2008.04.020. PMID 18456407.
- ↑ Roth, Mark B.; Nystul, Todd (1 June 2005). "Buying Time in Suspended Animation". Scientific American.
- ↑ Li, Jia; Zhang, Gencheng; Cai, Sally; Redington, Andrew N. (January 2008). "Effect of inhaled hydrogen sulfide on metabolic responses in anesthetized, paralyzed, and mechanically ventilated piglets". Pediatric Critical Care Medicine. 9 (1): 110–112. doi:10.1097/01.PCC.0000298639.08519.0C. PMID 18477923. Retrieved 2008-02-07.
H2S does not appear to have hypometabolic effects in ambiently cooled large mammals and conversely appears to act as a hemodynamic and metabolic stimulant.
- ↑ Haouzi, P.; Notet, V.; Chenuel, B.; Chalon, B; Sponne, I.; Ogier, V.; et al. (2008). "H2S induced hypometabolism in mice is missing in sedated sheep". Respir. Physiol. Neurobiol. 160 (1): 109–115. doi:10.1016/j.resp.2007.09.001. PMID 17980679.
- ↑ "Mark Roth: Suspended animation is within our grasp".
- ↑ "IK-1001 (Sodium Sulfide (Na2S) for Injection) in Subjects With Acute ST-Segment Elevation Myocardial Infarction". ClinicalTrials.gov. 2010-11-04.
This study has been withdrawn prior to enrollment. ( Company decision. Non-safety related )
- ↑ "Reduction of Ischemia-Reperfusion Mediated Cardiac Injury in Subjects Undergoing Coronary Artery Bypass Graft Surgery". ClinicalTrials.gov. 2011-08-03.
This study has been terminated. ( Study Terminated - Company decision )
- ↑ Barton, Larry L.; Fardeau, Marie-Laure; Fauque, Guy D. (2014). "Chapter 10. Hydrogen Sulfide: A Toxic Gas Produced by Dissimilatory Sulfate and Sulfur Reduction and Consumed by Microbial Oxidation". In Kroneck, Peter M.H.; Sosa Torres, Martha E. The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment. Metal Ions in Life Sciences. 14. Springer. pp. 237–277. doi:10.1007/978-94-017-9269-1_10.
- ↑ Jørgensen, B. B.; Nelson, D. C. (2004). "Sulfide oxidation in marine sediments: Geochemistry meets microbiology". In Amend, J. P.; Edwards, K. J.; Lyons, T. W. Sulfur Biogeochemistry – Past and Present. Geological Society of America. pp. 36–81.
- ↑ "The very first surface organism can be characterized as a catalyst for accelerating the formation of pyrite by providing a catalytic pathway for the flow of electrons from hydrogen sulfide to carbon dioxide." Wächtershäuser, Günter (1988-12-01). "Before enzymes and templates: theory of surface metabolism". Microbiol. Mol. Biol. Rev. 52 (4): 452–84. PMC 373159
 . PMID 3070320. Retrieved July 25, 2015.
. PMID 3070320. Retrieved July 25, 2015. - 1 2 "Impact From the Deep" in the October 2006 issue of Scientific American
- 1 2 Tobler, M; Riesch, R.; García de León, F. J.; Schlupp, I.; Plath, M. (2008). "Two endemic and endangered fishes, Poecilia sulphuraria (Álvarez, 1948) and Gambusia eurystoma Miller, 1975 (Poeciliidae, Teleostei) as only survivors in a small sulphidic habitat". Journal of Fish Biology. 72: 523–533. doi:10.1111/j.1095-8649.2007.01716.x.
- ↑ Palacios, Maura; Arias-Rodríguez, Lenín; Plath, Martin; Eifert, Constanze; Lerp, Hannes; Lamboj, Anton; Voelker, Gary; Tobler, Michael (2013). "The Rediscovery of a Long Described Species Reveals Additional Complexity in Speciation Patterns of Poeciliid Fishes in Sulfide Springs.". PLoS ONE. 8 (8): e71069. doi:10.1371/journal.pone.0071069.
- ↑ Bernardino, Angelo F.; Levin, Lisa A.; Thurber, Andrew R.; Smith, Craig R. (2012). "Comparative Composition, Diversity and Trophic Ecology of Sediment Macrofauna at Vents, Seeps and Organic Falls.". PLoS ONE. 7 (4): e33515. doi:10.1371/journal.pone.0033515.
- ↑ "Hydrothermal Vents". Marine Society of Australia. Retrieved 28 December 2014.
Additional resources
- Committee on Medical and Biological Effects of Environmental Pollutants (1979). Hydrogen Sulfide. Baltimore: University Park Press. ISBN 0-8391-0127-9.
- Siefers, Andrea (2010). A novel and cost-effective hydrogen sulfide removal technology using tire derived rubber particles (MS thesis). Iowa State University. Retrieved 8 February 2013.
External links
| Wikimedia Commons has media related to Hydrogen sulfide. |
- International Chemical Safety Card 0165
- Concise International Chemical Assessment Document 53
- National Pollutant Inventory - Hydrogen sulfide fact sheet
- NIOSH Pocket Guide to Chemical Hazards
- NACE (National Association of Corrosion Epal)