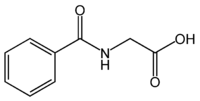
Hippuric acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Benzoylaminoethanoic acid | |
| Other names
Hippuric acid, N-benzoylglycine, benzoyl glycocoll, benzoyl amidoacetic acid | |
| Identifiers | |
| 495-69-2 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:18089 |
| ChEMBL | ChEMBL461 |
| ChemSpider | 451 |
| ECHA InfoCard | 100.007.098 |
| KEGG | C01586 |
| PubChem | 464 |
| UNII | TE0865N2ET |
| |
| |
| Properties | |
| C9H9NO3 | |
| Molar mass | 179.17 g/mol |
| Density | 1,371 g/cm3 |
| Melting point | 187 to 188 °C (369 to 370 °F; 460 to 461 K) |
| Boiling point | 240 °C (464 °F; 513 K) (decomposes) |
| Hazards | |
| Safety data sheet | Material Safety Data Sheet |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Hippuric acid (Gr. hippos, horse, ouron, urine) is a carboxylic acid found in the urine of horses and other herbivores. Hippuric acid crystallizes in rhombic prisms which are readily soluble in hot water, melt at 187 °C, and decompose at about 240 °C. High concentrations of hippuric acid may also indicate a toluene intoxication; however, scientists have called this correlation into question because there are other variables that influence levels of hippuric acid.[1] When many aromatic compounds such as benzoic acid and toluene are taken internally, they are converted to hippuric acid by reaction with the amino acid, glycine.
Synthesis
A modern synthesis of hippuric acid involves the acylation of glycine with benzoyl chloride:[2]
Reactions
Hippuric acid is readily hydrolysed by hot caustic alkalis to benzoic acid and glycine. Nitrous acid converts it into benzoyl glycolic acid, C6H5C(=O)OCH2CO2H. Its ethyl ester reacts with hydrazine to form hippuryl hydrazine, C6H5CONHCH2CONHNH2, which was used by Theodor Curtius for the preparation of hydrazoic acid.
History
Justus von Liebig showed in 1829 that hippuric acid differed from benzoic acid,[3] and in 1834 determined its constitution,[4] while in 1853 French chemist Victor Dessaignes (1800–1885) synthesized it by the action of benzoyl chloride on the zinc salt of glycine.[5] It was also formed by heating benzoic anhydride with glycine,[6] and by heating benzamide with monochloroacetic acid.
See also
References
- ↑ Pero, RW (2010). "Health consequences of catabolic synthesis of hippuric acid in humans". Current clinical pharmacology. 5 (1): 67–73. doi:10.2174/157488410790410588. PMID 19891605.
- ↑ A. W. Ingersoll and S. H. Babcock. "Hippuric acid". Org. Synth.; Coll. Vol., 2, p. 0328
- ↑ Liebig, Justus (1829) "Ueber die Säure, welche in dem Harn der grasfressenden vierfüssigen Thiere enthalten ist" (On the acid which is contained in the urine of grass-eating, four-footed animals), Annalen der Physik und Chemie, 17 : 389–399.
- ↑ Liebig, Justus (1834) "Ueber die Zusammensetzung der Hippursäure" (On the composition of hippuric acid), Annalen der Physik und Chemie, 32 : 573–574.
- ↑ Dessaignes V. (1853). "Ueber die Regeneration der Hippursäure" [On the regeneration of hippuric acid]. Annalen der Chemie und Pharmacie. 87 (3): 325–327. doi:10.1002/jlac.18530870311. See also: Dessaignes (1853) "Note sur la régénération de l'acide hipparique," Comptes rendus, 37 : 251–252.
- ↑ Curtius T. (1884). "Synthese von Hippursäure und Hippursäureäthern" [Synthesis of hippuric acid and hippuric acid esters]. Berichte der deutschen chemischen Gesellschaft. 17 (2): 1662–1663. doi:10.1002/cber.18840170225.
 This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "Hippuric Acid". Encyclopædia Britannica (11th ed.). Cambridge University Press.
This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "Hippuric Acid". Encyclopædia Britannica (11th ed.). Cambridge University Press.
