Harmala alkaloid

Several alkaloids that function as monoamine oxidase inhibitors (MAOIs) are found in the seeds of Peganum harmala (also known as Harmal or Syrian Rue), as well as tobacco leaves including harmine, harmaline, and harmalol, which are members of a group of substances with a similar chemical structure collectively known as harmala alkaloids. These alkaloids are of interest for their use in Amazonian shamanism, where they are derived from other plants. The harmala alkaloid harmine, once known as telepathine and banisterine, is a naturally occurring beta-carboline alkaloid that is structurally related to harmaline, and also found in the vine Banisteriopsis caapi. Tetrahydroharmine is also found in B. caapi and P. harmala. Dr. Alexander Shulgin has suggested that harmine may be a breakdown product of harmaline.[1] Harmine and harmaline are reversible MAOIs of the MAO-A isoform of the enzyme, and can stimulate the central nervous system by inhibiting the metabolism of monoamine compounds such as serotonin and norepinephrine.
The harmala alkaloids occur in Peganum harmala in concentrations of roughly 3%, though tests have documented anywhere from 2-7% or even higher,[2] as natural sources tend to vary widely in chemical makeup. Harmala alkaloids are also found in the Banisteriopsis caapi vine, the key plant ingredient in the sacramental beverage Ayahuasca, in concentrations that range between 0.31-8.43% for harmine, 0.03-0.83% for harmaline and 0.05-2.94% for tetrahydroharmine.[3] Although other psychoactive plants are occasionally added to Ayahuasca to achieve visionary states of consciousness, the recipes vary greatly and no single combination is common. Peganum harmala, normally consumed as a tea or used as an incense, is mentioned in classical Persian literature both as a sacred sacrament and as a medicine. The harmala alkaloids are not especially psychedelic, even at higher dosages, when hypnagogic visions, alongside vomiting and diarrhea, become the main effect.
Harmala alkaloids are also found in many other plants, such as passion flower. The leaves of P. incarnata have been reported variously to give 0.005%, 0.12 mg%, and nil, of harman alkaloids.[4]
Telepathine
Telepathine was originally thought to be the active chemical constituent of Banisteriopsis caapi, a key plant ingredient in the preparation of ayahuasca; a sacramental beverage from the Amazon. This isolated chemical was so named because of the reported effects of Ayahuasca among the indigenous users, including: collective contact with and/or visions of jaguars, snakes, and jeweled birds, and ancestral spirits; the ability to see future events; and as the name suggests, telepathic communication among tribal members. It was assumed to be a newly discovered chemical at the time, however, it was soon realized that Telepathine was already more widely known as "harmine" from its previous discovery in Peganum harmala (Syrian Rue).
Uses

As mentioned above, some harmala alkaloids can be used as an monoamine oxidase inhibitor (MAOI) to facilitate the ingestion of DMT and other tryptamines; while not generally used as a hallucinogen alone, there are reports of such use.[5] In high doses, it acts as purgative. Harmala alkaloids from Banisteriopsis caapi have been used to treat Parkinson's disease. As a benzodiazepine site inverse agonist, harmala alkaloids are used as a model for Essential Tremor (ET) when injected to animals. Rats being treated with harmaline exhibit severe tremors after 5–7 minutes. Individuals diagnosed with Essential Tremor have been found to have elevated blood levels of harmala alkaloids.[6]
Unlike many synthetic pharmaceutical MAOIs such as phenelzine, harmine is reversible and selective meaning it does not have nearly as high a risk for the "cheese syndrome" caused by consuming tyramine-containing foods, which is a risk associated with monoamine oxidase A inhibitors, but not monoamine oxidase B inhibitors.[7] Both MAO-A and MAO-B break down tyramine, but large doses of harmala alkaloids begin to affect MAO-B as well.
Anticancer
Isolated harmine was found to exhibit a cytotoxic effect on HL60 and K562 leukemic cell lines. This action might explain the previously observed cytotoxic effect of P. harmala on these cancer cells."[8]
Neuroprotective
Norharmane exerts neuroprotective properties by suppressing kynurenine neurotoxic metabolites such as quinolinic acid, 3OH-kynurenine and nitric oxide synthase.[9]
Legal Status
Australia
Harmala alkaloids are considered Schedule 9 prohibited substances under the Poisons Standard (October 2015).[10] A Schedule 9 substance is a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities.[10]
Exceptions are made when in herbs, or preparations, for therapeutic use such as : (a) containing 0.1 per cent or less of harmala alkaloids; or (b) in divided preparations containing 2 mg or less of harmala alkaloids per recommended daily dose.[10]
Chemical forms
- Harmine: C13H12N2O
- 7-Methoxy-1-methyl-9H-pyrido[3,4-b]indole
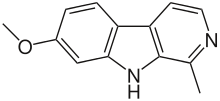
Harmine is a reversible inhibitor of MAO-A (RIMA).
- Harmaline: C13H14N2O
- 4,9-Dihydro-7-methoxy-1-methyl-3H-pyrido[3,4-b]indole
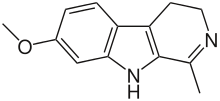
Harmaline is a reversible inhibitor of MAO-A (RIMA).[11]
- Harmalol: C12H12N2O
- 1-Methyl-4,9-dihydro-3H-pyrido[3,4-b]indol-7-ol

- Tetrahydroharmine: C13H16N2O
- 1,2,3,4-tetrahydro-harmine
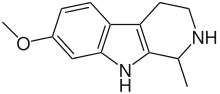
- Harmalan: C12H10N2
- 1-Methyl-3,4-dihydro-beta-carboline. Harmalan occurs in foodstuffs.[12]

- Harmine acid: methylester:
- Methyl-7-methoxy-beta-carboline-1-carboxylate
- Harmilinic acid:
- 7-Methoxy-3,4-dihydro-beta--carboline1-carboxylic acid
- Harmanamide:
- 1-Carbamoyl-7-methoxy-beta-carboline
- Acetylnorharmine:
- 1-Acetyl-7-methoxy-beta-carboline
See also
- Harmane
- Beta-carboline (norharmane)
- Monoamine oxidase inhibitor
- Reversible inhibitor of monoamine oxidase A (RIMA)
References
- ↑ http://www.erowid.org/library/books_online/tihkal/tihkal13.shtml
- ↑ Herraiz T, González D, Ancín-Azpilicueta C, Arán VJ, Guillén H (2010). "beta-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO)". Food Chem Toxicol. 48 (3): 839–43. doi:10.1016/j.fct.2009.12.019. PMID 20036304.
- ↑ Callaway JC, Brito GS & Neves ES (2005). Phytochemical analyses of Banisteriopsis caapi and Psychotria viridis Journal of Psychoactive Drugs 37(2): 145-150.
- ↑ Kamaldeep Dhawan, Sanju Dhawan, Anupam Sharma, Passiflora: a review update, Journal of Ethnopharmacology, Volume 94, Issue 1, September 2004, Pages 1-23, ISSN 0378-8741, 10.1016/j.jep.2004.02.023.
- ↑ Shulgin, Alexander. "#13 Harmaline", Erowid Online Texts: TiHKAL #13 HARMALINE, retrieved November 26, 2006.
- ↑ Louis ED; Zheng, W; Jurewicz, EC; Watner, D; Chen, J; Factor-Litvak, P; Parides, M (2002). "Elevation of blood beta-carboline alkaloids in essential tremor.". Neurology. 59 (12): 1940–4. doi:10.1212/01.wnl.0000038385.60538.19. PMID 12499487.
- ↑ McKenna, Callaway, & Grb. "Scientific Investigation of Ayahuasca", Scientific Investigation of Ayahuasca, retrieved 2007-06-03.
- ↑ Jahaniani, F; Ebrahimi, SA; Rahbar-Roshandel, N; Mahmoudian, M (July 2005). "Xanthomicrol is the main cytotoxic component of Dracocephalum kotschyii and a potential anti-cancer agent". Phytochemistry. 66 (13): 1581–92. doi:10.1016/j.phytochem.2005.04.035. PMID 15949825.
- ↑ Chiarugi A, Dello Sbarba P, Paccagnini A, Donnini S, Filippi S, Moroni F (August 2000). "Combined inhibition of indoleamine 2,3-dioxygenase and nitric oxide synthase modulates neurotoxin release by interferon-gamma-activated macrophages". J. Leukoc. Biol. 68 (2): 260–6. PMID 10947071.
- 1 2 3 Poisons Standard October 2015 https://www.comlaw.gov.au/Details/F2015L01534
- ↑ Edward J. Massaro, Handbook of Neurotoxicology
- ↑ Herraiz T (2004). "Relative exposure to beta-carbolines norharman and harman from foods and tobacco smoke". Food Addit Contam. 21 (11): 1041–50. doi:10.1080/02652030400019844. PMID 15764332.