Copolymer
A polymer derived from more than one species of monomer.
Note: Copolymers that are obtained by copolymerization of two monomer species
are sometimes termed bipolymers, those obtained from three monomers terpolymers,
those obtained from four monomers quaterpolymers, etc.
[1]
Alternating copolymers: A copolymer consisting of macromolecules comprising
two species of monomeric units in alternating sequence.
Note: An alternating copolymer may be considered as a homopolymer derived from
an implicit or hypothetical monomer.[1]
Block copolymers: A portion of a macromolecule, comprising many constitutional
units, that has at least one feature which is not present in the adjacent portions.[1]
Graft macromolecule: A macromolecule with one or more species of
block connected to the main chain as side-chains, these side-chains having constitutional
or configurational features that differ from those in the main chain.[2]
.jpg)
When two or more different monomers unite together to polymerize, their result is called a copolymer and its process is called copolymerization.
Commercially relevant copolymers include acrylonitrile butadiene styrene (ABS), styrene/butadiene co-polymer (SBR), nitrile rubber, styrene-acrylonitrile, styrene-isoprene-styrene (SIS) and ethylene-vinyl acetate.
Types of copolymers

Since a copolymer consists of at least two types of constituent units (also structural units), copolymers can be classified based on how these units are arranged along the chain.[3] These include:
- Alternating copolymers with regular alternating A and B units (2)
- Periodic copolymers with A and B units arranged in a repeating sequence (e.g. (A-B-A-B-B-A-A-A-A-B-B-B)n)
- Statistical copolymers are copolymers in which the sequence of monomer residues follows a statistical rule. If the probability of finding a given type monomer residue at a particular point in the chain is equal to the mole fraction of that monomer residue in the chain, then the polymer may be referred to as a truly random copolymer[4] (3).
- Block copolymers comprise two or more homopolymer subunits linked by covalent bonds (4). The union of the homopolymer subunits may require an intermediate non-repeating subunit, known as a junction block. Block copolymers with two or three distinct blocks are called diblock copolymers and triblock copolymers, respectively.
Copolymers may also be described in terms of the existence of or arrangement of branches in the polymer structure. Linear copolymers consist of a single main chain whereas branched copolymers consist of a single main chain with one or more polymeric side chains.
Other special types of branched copolymers include star copolymers, brush copolymers, and comb copolymers. In gradient copolymers the monomer composition changes gradually along the chain.
A terpolymer is a copolymer consisting of three distinct monomers. The term is derived from ter (Latin), meaning thrice, and polymer.
- Stereoblock copolymers
A special structure can be formed from one monomer where now the distinguishing feature is the tacticity of each block.
Graft copolymers
Graft copolymers are a special type of branched copolymer in which the side chains are structurally distinct from the main chain. The illustration (5) depicts a special case where the main chain and side chains are composed of distinct homopolymers. However, the individual chains of a graft copolymer may be homopolymers or copolymers. Note that different copolymer sequencing is sufficient to define a structural difference, thus an A-B diblock copolymer with A-B alternating copolymer side chains is properly called a graft copolymer.
For example, suppose we perform a free-radical polymerization of styrene in the presence of polybutadiene, a synthetic rubber, which retains one reactive C=C double bond per residue. We get polystyrene chains growing out in either direction from some of the places where there were double bonds, with a one-carbon rearrangement. Or to look at it the other way around, the result is a polystyrene backbone with polybutadiene chains growing out of it in both directions. This is an interesting copolymer variant in that one of the ingredients was a polymer to begin with.
As with block copolymers, the quasi-composite product has properties of both "components". In the example cited, the rubbery chains absorb energy when the substance is hit, so it is much less brittle than ordinary polystyrene. The product is called high-impact polystyrene, or HIPS.
Block copolymers
One kind of copolymer is called a "block copolymer". Block copolymers are made up of blocks of different polymerized monomers and is usually made by first polymerizing styrene, and then subsequently polymerizing methyl methacrylate (MMA) from the reactive end of the polystyrene chains. This polymer is a "diblock copolymer" because it contains two different chemical blocks. Triblocks, tetrablocks, multiblocks, etc. can also be made. Diblock copolymers are made using living polymerization techniques, such as atom transfer free radical polymerization (ATRP), reversible addition fragmentation chain transfer (RAFT), ring-opening metathesis polymerization (ROMP), and living cationic or living anionic polymerizations.[5] An emerging technique is chain shuttling polymerization.
The "blockiness" of a copolymer is a measure of the adjacency of comonomers vs their statistical distribution. Many or even most synthetic polymers are in fact copolymers, containing about 1-20% of a minority monomoner. In such cases, blockiness is undesirable.[6]
Phase separation

Block copolymers are interesting because they can "microphase separate" to form periodic nanostructures, as in the styrene-butadiene-styrene block copolymer shown at right. The polymer is known as Kraton and is used for shoe soles and adhesives. Owing to the microfine structure, the transmission electron microscope or TEM was needed to examine the structure. The butadiene matrix was stained with osmium tetroxide to provide contrast in the image. The material was made by living polymerization so that the blocks are almost monodisperse, so helping to create a very regular microstructure. The molecular weight of the polystyrene blocks in the main picture is 102,000; the inset picture has a molecular weight of 91,000, producing slightly smaller domains.
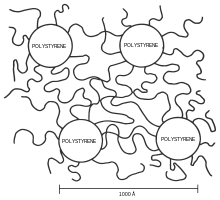
Microphase separation is a situation similar to that of oil and water. Oil and water are immiscible - they phase separate. Due to incompatibility between the blocks, block copolymers undergo a similar phase separation. Because the blocks are covalently bonded to each other, they cannot demix macroscopically as water and oil. In "microphase separation" the blocks form nanometer-sized structures. Depending on the relative lengths of each block, several morphologies can be obtained. In diblock copolymers, sufficiently different block lengths lead to nanometer-sized spheres of one block in a matrix of the second (for example PMMA in polystyrene). Using less different block lengths, a "hexagonally packed cylinder" geometry can be obtained. Blocks of similar length form layers (often called lamellae in the technical literature). Between the cylindrical and lamellar phase is the gyroid phase. The nanoscale structures created from block copolymers could potentially be used for creating devices for use in computer memory, nanoscale-templating and nanoscale separations.[7]
Polymer scientists use thermodynamics to describe how the different blocks interact.[8][9] The product of the degree of polymerization, n, and the Flory-Huggins interaction parameter, , gives an indication of how incompatible the two blocks are and whether or not they will microphase separate. For example, a diblock copolymer of symmetric composition will microphase separate if the product is greater than 10.5. If is less than 10.5, the blocks will mix and microphase separation is not observed. The incompatibility between the blocks also affects the solution behavior of these copolymers and their adsorption behavior on various surfaces.[10]
Copolymer equation
An alternating copolymer has the formula: -A-B-A-B-A-B-A-B-A-B-, or -(-A-B-)n-. The molar ratios of the monomer in the polymer is close to one, which happens when the reactivity ratios r1 & r2 are close to zero, as given by the Mayo–Lewis equation also called the copolymerization equation:[11]
where r1 = k11/k12 & r2 = k22/k21
Copolymer engineering
Copolymerization is used to modify the properties of manufactured plastics to meet specific needs, for example to reduce crystallinity, modify glass transition temperature or to improve solubility. It is a way of improving mechanical properties, in a technique known as rubber toughening. Elastomeric phases within a rigid matrix act as crack arrestors, and so increase the energy absorption when the material is impacted for example. Acrylonitrile butadiene styrene is a common example.
See also
References
- 1 2 3 McNaught, A. D.; Wilkinson, A. (1996). "Glossary of basic terms in polymer science (IUPAC Recommendations 1996)". Pure and Applied Chemistry. 68: 2287–2311. doi:10.1351/goldbook.C01335.
- ↑ "Glossary of basic terms in polymer science (IUPAC Recommendations 1996)" (PDF). Pure and Applied Chemistry. 68 (12): 2287–2311. 1996. doi:10.1351/pac199668122287.
- ↑ Jenkins, A. D.; Kratochvíl, P.; Stepto, R. F. T.; Suter, U. W. (1996). "Glossary of Basic Terms in Polymer Science". Pure Appl. Chem. 68 (12): 2287–2311. doi:10.1351/pac199668122287.
- ↑ Painter P. C. and Coleman M. M., Fundamentals of Polymer Science, CRC Press, 1997, p 14.
- ↑ Hadjichristidis N., Pispas S., Floudas G. Block copolymers: synthetic strategies, physical properties, and applications – Wiley, 2003.
- ↑ Chum, P. S.; Swogger, K. W., "Olefin Polymer Technologies-History and Recent Progress at the Dow Chemical Company", Progress in Polymer Science 2008, volume 33, 797-819. doi:10.1016/j.progpolymsci.2008.05.003
- ↑ Gazit, Oz; Khalfin, Rafail; Cohen, Yachin; Tannenbaum, Rina (2009). "Self-assembled diblock copolymer "nanoreactors" as catalysts for metal nanoparticle synthesis". Journal of Physical Chemistry C. 113: 576–583. doi:10.1021/jp807668h.
- ↑ Bates, Frank S.; Fredrickson, Glenn H. (2014). "Block Copolymer Thermodynamics: Theory and Experiment". Annual Review of Physical Chemistry. 41: 525–557. doi:10.1146/annurev.pc.41.100190.002521.
- ↑ Chremos, Alexandros; Nikoubashman, Arash; Panagiotopoulos, Athanassios (2014). "Flory-Huggins parameter χ, from binary mixtures of Lennard-Jones particles to block copolymer melts". J. Chem. Phys. 140: 054909. doi:10.1063/1.4863331.
- ↑ Hershkovitz, Eli; Tannenbaum, Allen; Tannenbaum, Rina (2008). "Adsorption of block co-polymers from selective solvents on curved surfaces". Macromolecules. 41: 3190–3198. doi:10.1021/ma702706p.
- ↑ Mayo, Frank R.; Lewis, Frederick M. (1944). "Copolymerization. I. A Basis for Comparing the Behavior of Monomers in Copolymerization; The Copolymerization of Styrene and Methyl Methacrylate". J. Am. Chem. Soc. 66 (9): 1594–1601. doi:10.1021/ja01237a052.
External links
| Wikimedia Commons has media related to Copolymers. |