Glycine receptor
The glycine receptor, or GlyR, is the receptor for the amino acid neurotransmitter glycine. GlyR is an ionotropic receptor that produces its effects through chloride current. It is one of the most widely distributed inhibitory receptors in the central nervous system and has important roles in a variety of physiological processes, especially in mediating inhibitory neurotransmission in the spinal cord and brainstem.[1]
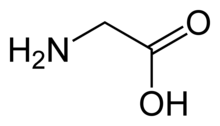
The receptor can be activated by a range of simple amino acids including glycine, β-alanine and taurine, and can be selectively blocked by the high-affinity competitive antagonist strychnine.[2] Caffeine is a competitive antagonist of GlyR.[3]
Gephyrin has been shown to be necessary for GlyR clustering at inhibitory synapses.[4][5] GlyR is known to colocalize with the GABAA receptor on some hippocampal neurons.[4] Nevertheless, some exceptions can occur in the central nervous system where the GlyR α1 subunit and gephyrin, its anchoring protein, are not found in dorsal root ganglion neurons despite the presence of GABAA receptors.[6]
Arrangement of subunits
Strychnine-sensitive GlyRs are members of a family of ligand-gated ion channels. Receptors of this family are arranged as five subunits surrounding a central pore, with each subunit composed of four α helical transmembrane segments.[7] There are presently four known isoforms of the α-subunit (α1-4) of GlyR that are essential to bind ligands (GLRA1, GLRA2, GLRA3, GLRA4) and a single β-subunit (GLRB).
The adult form of the GlyR is the heteromeric α1β receptor, which is believed to have a stoichiometry (proportion) of three α1 subunits and two β subunits [8] or four α1 subunits and one β subunit.[9] The α-subunits are also able to form functional homo-pentameric receptors in heterologous expression systems in African clawed frog's oocytes or mammalian cell lines,[9] and the α1 homomeric receptor is essential for studies of channel pharmacokinetics and pharmacodynamics.[10]
Glycine receptors in diseases
Disruption of GlyR surface expression or reduced ability of expressed GlyRs to conduct chloride ions results in the rare neurological disorder, hyperekplexia. The disorder is characterized by an exaggerated response to unexpected stimuli which is followed by a temporary but complete muscular rigidity often resulting in an unprotected fall. Chronic injuries as a result of the falls are symptomatic of the disorder.[1] A mutation in GLRA1 is responsible for some cases of stiff person syndrome.[11]
Ligands
Agonists
- β-Alanine
- D-Alanine
- D-Serine
- Glycine
- Hypotaurine
- L-Alanine
- L-Proline
- L-Serine
- Milacemide
- Quisqualamine
- Sarcosine
- Taurine
PAMs
Antagonists
References
- 1 2 Lynch JW (October 2004). "Molecular structure and function of the glycine receptor chloride channel". Physiological reviews. 84 (4): 1051–95. doi:10.1152/physrev.00042.2003. PMID 15383648.
- ↑ Rajendra, S; Lynch JW; Schofield PR (1997). "The glycine receptor". Pharmacology and Therapeutics. 73 (2): 121–146. doi:10.1016/S0163-7258(96)00163-5. PMID 9131721.
- ↑ Duan, L; Yang, J; Slaughter, MM (2009). "Caffeine inhibition of ionotropic glycine receptors". J Physiol. 587 (16): 4063–75. doi:10.1113/jphysiol.2009.174797. PMC 2756438
 . PMID 19564396.
. PMID 19564396. - 1 2 Levi, S.; Logan, S. M.; Tovar, K. R.; Craig, A. M. (2004). "Gephyrin is Critical for Glycine Receptor Clustering but Not for the Formation of Functional GABAergic Synapses in Hippocampal Neurons". Journal of Neuroscience. 24 (1): 207–217. doi:10.1523/JNEUROSCI.1661-03.2004. PMID 14715953.
- ↑ Feng G, Tintrup H, Kirsch J, et al. (November 1998). "Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity". Science. 282 (5392): 1321–4. Bibcode:1998Sci...282.1321F. doi:10.1126/science.282.5392.1321. PMID 9812897.
- ↑ Lorenzo LE, Godin AG, Wang F, St-Louis M, Carbonetto S, Wiseman PW, Ribeiro-da-Silva A, De Koninck Y (June 2014). "Gephyrin Clusters Are Absent from Small Diameter Primary Afferent Terminals Despite the Presence of GABAA Receptors". J. Neurosci. 34 (24): 8300–17. doi:10.1523/JNEUROSCI.0159-14.2014. PMID 24920633.
- ↑ Miyazawa, A; Fujiyoshi Y; Unwin N (2003). "Structure and gating mechanism of the acetylcholine receptor pore". Nature. 423 (6943): 949–955. Bibcode:2003Natur.423..949M. doi:10.1038/nature01748. PMID 12827192.
- ↑ Kuhse, J; Laube B; Magalei D; Betz H (1993). "Assembly of the inhibitory glycine receptor: identification of amino acid sequence motifs governing subunit stoichiometry". Neuron. 11 (6): 1049–1056. doi:10.1016/0896-6273(93)90218-G. PMID 8274276.
- 1 2 Kuhse, J; Betz H; Kirsch J (1995). "The inhibitory glycine receptor: architecture, synaptic localization and molecular pathology of a postsynaptic ion-channel complex". Current Opinion in Neurobiology. 5 (3): 318–323. doi:10.1016/0959-4388(95)80044-1. PMID 7580154.
- ↑ Lewis, TM; Schofield PR; McClellan AM (2003). "Kinetic determinants of agonist action at the recombinant human glycine receptor". Journal of Physiology. 549 (Part 2): 361–374. doi:10.1113/jphysiol.2002.037796. PMC 2342959
 . PMID 12679369. Retrieved 2007-01-16.
. PMID 12679369. Retrieved 2007-01-16. - ↑ Online Mendelian Inheritance in Man (OMIM) STIFF-PERSON SYNDROME; SPS -184850
External links
- Glycine Receptors at the US National Library of Medicine Medical Subject Headings (MeSH)