Extracorporeal membrane oxygenation
| Extracorporeal membrane oxygenation | |
|---|---|
| Intervention | |
_ECMO_for_cardiac_or_respiratory_failure.jpg) | |
| ICD-10-PCS | Z92.81 |
| ICD-9-CM | 39.65 |
| MeSH | 29295 |
| MedlinePlus | 007234 |
| HCPCS-L2 | 36822 |
Extracorporeal membrane oxygenation (ECMO), also known as extracorporeal life support (ECLS) ,is an extracorporeal technique of providing both cardiac and respiratory support to persons whose heart and lungs are unable to provide an adequate amount of gas exchange to sustain life.
This intervention has mostly been used on children, but it is seeing more use in adults with cardiac and respiratory failure. ECMO works by removing blood from the person's body and artificially removing the carbon dioxide and oxygenating red blood cells. Generally it is only used in the later treatment of a person with heart or lung failure as it is solely a life-sustaining intervention. Cardiopulmonary bypass is generally used for shorter-term treatment.
Medical uses
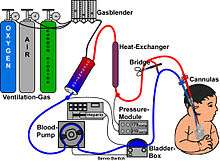
Guidelines that describe the indications and practice of ECMO are published by the Extracorporeal Life Support Organization (ELSO). Criteria for the initiation of ECMO include acute severe cardiac or pulmonary failure that is potentially reversible and unresponsive to conventional management. Examples of clinical situations that may prompt the initiation of ECMO include the following:[1]
- Hypoxemic respiratory failure with a ratio of arterial oxygen tension to fraction of inspired oxygen (PaO2/FiO2) of <100 mmHg despite optimization of the ventilator settings, including the fraction of inspired oxygen (FiO2), positive end-expiratory pressure (PEEP), and inspiratory to expiratory (I:E) ratio
- Hypercapnic respiratory failure with an arterial pH <7.20
- Refractory cardiogenic shock
- Cardiac arrest
- Failure to wean from cardiopulmonary bypass after cardiac surgery
- As a bridge to either heart transplantation or placement of a ventricular assist device
In those with cardiac arrest or cardiogenic shock it appears to improve survival and good outcomes.[2]
Outcomes
A registry of patients that have received ECMO is maintained by the Extracorporeal Life Support Organization (ELSO). The last publication of ELSO registry data reported outcomes on nearly 51,000 patients with 75% survival for neonatal respiratory failure, 56% survival for pediatric respiratory failure, and 55% survival for adult respiratory failure.[3] With acute respiratory failure use of ECMO has been shown to improve survival rates.[4][5] Survival rates from 50 to 70 percent[6] have been reported in observational and uncontrolled clinical trials.[7] The survival rates reported are better than historical survival rates.[8][9][10] In the United Kingdom, respiratory (VV) ECMO is concentrated in designated ECMO centres to ensure top-quality care.
Contraindications
Most contraindications are relative, balancing the risks of the procedure (including the risk of using valuable resources that could be used for others) versus the potential benefits. The relative contraindications are:
- Conditions incompatible with normal life if the person recovers
- Preexisting conditions that affect the quality of life (CNS status, end-stage malignancy, risk of systemic bleeding with anticoagulation)
- Age and size
- Futility: those who are too sick, have been on conventional therapy too long, or have a fatal diagnosis.
Types
_ECMO_for_cardiac_or_respiratory_failure.jpg)
_ECMO_for_isolated_respiratory_failure.jpg)
There are several forms of ECMO, the two most common of which are the veno-arterial (VA) and veno-venous (VV). In both modalities, blood drained from the venous system is oxygenated outside of the body. In VA ECMO, this blood is returned to the arterial system and in VV ECMO the blood is returned to the venous system. In VV ECMO, no cardiac support is provided.
Veno-arterial (VA)
In veno-arterial ECMO, a venous cannula is usually placed in the right common femoral vein for extraction and an arterial cannula is usually placed into the right femoral artery for infusion.[12] The tip of the femoral venous cannula should be maintained near the junction of the inferior vena cava and right atrium, while the tip of the femoral arterial cannula is maintained in the iliac artery.[12] In adults accessing the femoral artery is preferred because the insertion is simpler.[12] Central VA ECMO may be used if cardiopulmonary bypass has already been established (with cannulae in the right atrium and ascending aorta).
Veno-venous (VV)
In veno-venous ECMO cannulae are usually placed in the right common femoral vein for drainage and right internal jugular vein for infusion.[13] Alternatively, a dual-lumen catheter is inserted into the right internal jugular vein, draining blood from the superior and inferior vena cavae and returning it to the right atrium.
Initiation
ECMO should be performed only by clinicians with training and experience in its initiation, maintenance, and discontinuation. Once it has been decided that ECMO will be initiated, the person is anticoagulated with intravenous heparin and then the cannulae are inserted. ECMO support is initiated once the cannulae are connected to the appropriate limbs of the ECMO circuit.
Cannulation
Cannulae are usually placed percutaneously by the Seldinger technique. The largest cannulas that can be placed in the vessels are used in order to maximise flow and minimise pressures Bernoulli's equation.
ECMO required for complications of cardiac surgery can be placed directly into the appropriate chambers of the heart or great vessels.
Titration
Following cannulation they are connected to the ECMO circuit and the blood flow is increased until respiratory and hemodynamic status is stable.
Maintenance
Once the initial respiratory and hemodynamic goals have been achieved, the blood flow is maintained at that rate. Frequent assessment and adjustments are facilitated by continuous venous oximetry, which directly measures the oxyhemoglobin saturation of the blood in the venous limb of the ECMO circuit.
Special considerations
VV ECMO is typically used for respiratory failure, while VA ECMO is used for cardiac failure. There are unique considerations for each type of ECMO, which influence management.
Blood flow
Near-maximum flow rates are usually desired during VV ECMO to optimize oxygen delivery. In contrast, the flow rate used during VA ECMO must be high enough to provide adequate perfusion pressure and venous oxyhemoglobin saturation (measured on drainage blood) but low enough to provide sufficient preload to maintain left ventricular output.
Diuresis
Since most people are fluid-overloaded when ECMO is initiated, aggressive diuresis is warranted once the person is stable on ECMO. Ultrafiltration can be easily added to the ECMO circuit if the person has inadequate urine output.
Left ventricular monitoring
Left ventricular output is rigorously monitored during VA ECMO because left ventricular output can become worse.[14][15]
Weaning and discontinuing
For those with respiratory failure, improvements in radiographic appearance, pulmonary compliance, and arterial oxyhemoglobin saturation indicate that the person may be ready to be taken off of ECMO support. For those with cardiac failure, enhanced aortic pulsatility correlates with improved left ventricular output and indicates that they may be ready to be taken off of ECMO support. Once the decision has been made to discontinue ECMO, the cannulae are removed.
Veno-venous ECMO liberation trial
VV ECMO trials are performed by eliminating all countercurrent sweep gas through the oxygenator. Extracorporeal blood flow remains constant, but gas transfer does not occur. They are then observed for several hours, during which the ventilator settings that are necessary to maintain adequate oxygenation and ventilation off ECMO are determined as indicated by arterial and venous blood gas results.
Veno-arterial ECMO liberation trial
VA ECMO trials require temporary clamping of both the drainage and infusion lines, while allowing the ECMO circuit to circulate through a bridge between the arterial and venous limbs. This prevents thrombosis of stagnant blood within the ECMO circuit. In addition, the arterial and venous lines should be flushed continuously with heparinized saline or intermittently with heparinized blood from the circuit. In general, VA ECMO trials are shorter in duration than VV ECMO trials because of the higher risk of thrombus formation.
Complications
A common consequence in ECMO-treated adults is neurological injury, which may include subarachnoid hemorrhage, ischemic infarctions in susceptible areas of the brain, hypoxic-ischemic encephalopathy, unexplained coma, and brain death.[16] Bleeding occurs in 30 to 40 percent of those receiving ECMO and can be life-threatening. It is due to both the necessary continuous heparin infusion and platelet dysfunction. Meticulous surgical technique, maintaining platelet counts greater than 100,000/mm3, and maintaining the target ACT reduce the likelihood of bleeding. Heparin-induced thrombocytopenia (HIT) is increasingly common among people receiving ECMO. When HIT is suspected, the heparin infusion is usually replaced by a non-heparin anticoagulant.[17] There is retrograde blood flow in the descending aorta whenever the femoral artery and vein are used for VA ECMO. Stasis of the blood can occur if left ventricular output is not maintained, which may result in thrombosis. The CESAR study has shown an improvement in 6 month survival without severe disability in patients getting ECMO versus conventional ventilation in ARDS.[18] In VA ECMO, those whose cardiac function does not recover sufficiently to be weaned from ECMO may be bridged to a ventricular assist device (VAD) or transplant. A variety of complications can occur during cannulation, including vessel perforation with bleeding, arterial dissection, distal ischemia, and incorrect location (e.g., venous cannula placed within the artery), but these events occur highly infrequently.
In infants aged less than 34 weeks of gestation, several physiologic systems are not well-developed, especially the blood vessels of the brain and germinal matrix, resulting in high sensitivity to slight changes in pH, PaO2, and intracranial pressure.[19] Preterm infants are at unacceptably high risk for intraventricular hemorrhage (IVH) if administered ECMO at a gestational age less than 32 weeks.[20] Given the risk of IVH, it has become standard practice to perform a brain ultrasound prior to administering ECMO even in more mature neonatal patients.[19]
Research
A 2014 study showed that a factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk.[21] Experiments on neonatal animals showed that ECMO treatment can lead to apoptosis of enterocytes, damage of the intestinal mucosal barrier and bacterial translocation. This might explain greater severity of systemic inflammatory response syndrome in neonates.[22] ECMO has also seen its use on cadavers as being able to increase the viability rate of transplanted organs.[23]
References
- ↑ "General Guidelines for all ECLS Cases" (PDF). Extracorporeal Life Support Organization. Retrieved 2015-04-15.
- ↑ Ouweneel, DM; Schotborgh, JV; Limpens, J; Sjauw, KD; Engström, AE; Lagrand, WK; Cherpanath, TG; Driessen, AH; de Mol, BA; Henriques, JP (19 September 2016). "Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis.". Intensive care medicine. PMID 27647331.
- ↑ Paden, Matthew L.; Conrad, Steven A.; Rycus, Peter T.; Thiagarajan, Ravi R. (2013-01-01). "Extracorporeal Life Support Organization Registry Report 2012". ASAIO Journal. 59 (3): 202–210. doi:10.1097/mat.0b013e3182904a52.
- ↑ Peek, GJ; Moore, HM; Moore, N; Sosnowski, AW; Firmin, RK (1997). "Extracorporeal membrane oxygenation for adult respiratory failure". Chest. 112 (3): 759–64. doi:10.1378/chest.112.3.759. PMID 9315812.
- ↑ Lewandowski, K.; Rossaint, R.; Pappert, D.; Gerlach, H.; Slama, K.-J.; Weidemann, H.; Frey, D. J. M.; Hoffmann, O.; Keske, U. (1997). "High survival rate in 122 ARDS patients managed according to a clinical algorithm including extracorporeal membrane oxygenation". Intensive Care Medicine. 23 (8): 819–35. doi:10.1007/s001340050418. PMID 9310799.
- ↑ Hemmila, Mark R.; Rowe, Stephen A.; Boules, Tamer N.; Miskulin, Judiann; McGillicuddy, John W.; Schuerer, Douglas J.; Haft, Jonathan W.; Swaniker, Fresca; Arbabi, Saman (2004). "Extracorporeal Life Support for Severe Acute Respiratory Distress Syndrome in Adults". Annals of Surgery. 240 (4): 595–605; discussion 605–7. doi:10.1097/01.sla.0000141159.90676.2d. PMC 1356461
 . PMID 15383787.
. PMID 15383787. - ↑ Brogan, Thomas V.; Thiagarajan, Ravi R.; Rycus, Peter T.; Bartlett, Robert H.; Bratton, Susan L. (2009). "Extracorporeal membrane oxygenation in adults with severe respiratory failure: A multi-center database". Intensive Care Medicine. 35 (12): 2105–14. doi:10.1007/s00134-009-1661-7. PMID 19768656.
- ↑ Kolla, S; Awad, SS; Rich, PB; Schreiner, RJ; Hirschl, RB; Bartlett, RH (1997). "Extracorporeal life support for 100 adult patients with severe respiratory failure". Annals of Surgery. 226 (4): 544–64; discussion 565–6. doi:10.1097/00000658-199710000-00015. PMC 1191077
 . PMID 9351722.
. PMID 9351722. - ↑ Rich, PB; Awad, SS; Kolla, S; Annich, G; Schreiner, RJ; Hirschl, RB; Bartlett, RH (1998). "An approach to the treatment of severe adult respiratory failure". Journal of critical care. 13 (1): 26–36. doi:10.1016/S0883-9441(98)90026-0. PMID 9556124.
- ↑ Ullrich, R; Lorber, C; Röder, G; Urak, G; Faryniak, B; Sladen, RN; Germann, P (1999). "Controlled airway pressure therapy, nitric oxide inhalation, prone position, and extracorporeal membrane oxygenation (ECMO) as components of an integrated approach to ARDS". Anesthesiology. 91 (6): 1577–86. doi:10.1097/00000542-199912000-00007. PMID 10598597.
- 1 2 Van Meurs, Krisa; Lally, Kevin; Zwischenberger, Joseph B.; Peek, Giles, eds. (2005). ECMO: Extracorporeal Cardiopulmonary Support in Critical Care. Ann Arbor: Extracorporeal Life Support Organization. ISBN 978-0-9656756-2-8.
- 1 2 3 Madershahian, Navid; Nagib, Ragi; Wippermann, Jens; Strauch, Justus; Wahlers, Thorsten (2006). "A Simple Technique of Distal Limb Perfusion During Prolonged Femoro-Femoral Cannulation". Journal of Cardiac Surgery. 21 (2): 168–9. doi:10.1111/j.1540-8191.2006.00201.x. PMID 16492278.
- ↑ Wang, Dongfang; Zhou, Xiaoqin; Liu, Xiaojun; Sidor, Bill; Lynch, James; Zwischenberger, Joseph B. (2008). "Wang-Zwische Double Lumen Cannula—Toward a Percutaneous and Ambulatory Paracorporeal Artificial Lung". ASAIO Journal. 54 (6): 606–11. doi:10.1097/MAT.0b013e31818c69ab. PMID 19033774.
- ↑ Cohen, Gordon; Permut, Lester (2005). "Decision making for mechanical cardiac assist in pediatric cardiac surgery". Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual. 8: 41–50. doi:10.1053/j.pcsu.2005.02.004. PMID 15818357.
- ↑ Vural, Kerem M. (2008). "Ventricular assist device applications". Anadolu Kardiyoloji Dergisi. 8 (Suppl 2): 117–30. PMID 19028644.
- ↑ "Neurological Injury in Adults Treated With Extracorporeal Membrane Oxygenation". Archives of Neurology. 68: 1543. doi:10.1001/archneurol.2011.209. Retrieved 2013-08-08.
- ↑ Cornell, Timothy; Wyrick, Polly; Fleming, Geoffrey; Pasko, Deborah; Han, Yong; Custer, Joseph; Haft, Jonathan; Annich, Gail (2007). "A Case Series Describing the Use of Argatroban in Patients on Extracorporeal Circulation". ASAIO Journal. 53 (4): 460–3. doi:10.1097/MAT.0b013e31805c0d6c. PMID 17667231.
- ↑ "Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial". Lancet. 374 (9698): 1351–63. Oct 2009. doi:10.1016/S0140-6736(09)61069-2. PMID 19762075.
- 1 2 "Concepts of Neonatal ECMO". The Internet Journal of Perfusionists. 1 (2). 2001. doi:10.5580/d9.
- ↑ Jobe, Alan H. (2004). "Post-conceptional age and IVH in ECMO patients". The Journal of Pediatrics. 145 (2): A2. doi:10.1016/j.jpeds.2004.07.010.
- ↑ Larsson M, Rayzman V, Nolte MW, et al. (Jan 2014). "A Factor XIIa Inhibitory Antibody Provides Thromboprotection in Extracorporeal Circulation Without Increasing Bleeding Risk.". Sci Transl Med. 6 (222): 222. doi:10.1126/scitranslmed.3006804. PMID 24500405.
- ↑ MohanKumar K (Feb 2014). "Intestinal epithelial apoptosis initiates gut mucosal injury during extracorporeal membrane oxygenation in the newborn piglet". Lab Invest. 94 (2): 150–160. doi:10.1038/labinvest.2013.149. PMC 3946757
 . PMID 24365747.
. PMID 24365747. - ↑ Magliocca, JF; Magee, JC; Rowe, SA; Gravel, MT; Chenault Rh, 2nd; Merion, RM; Punch, JD; Bartlett, RH; Hemmila, MR (2005). "Extracorporeal support for organ donation after cardiac death effectively expands the donor pool". The Journal of trauma. 58 (6): 1095–101; discussion 1101–2. doi:10.1097/01.ta.0000169949.82778.df. PMID 15995454.