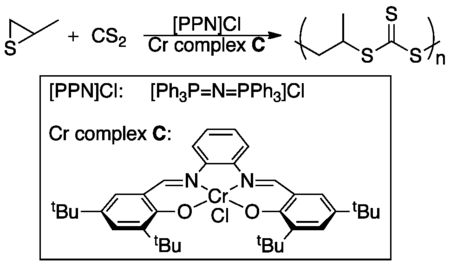Episulfide
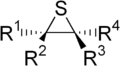
Episulfides are a class of organic compounds that contain a saturated heterocyclic ring consisting of two carbon atoms and one sulfur atom. It is the sulfur analogue of an epoxide or aziridine. They are also known as thiiranes, olefin sulfides, thioalkylene oxides, and thiacyclopropanes.
The parent episulfide is thiirane. Most preparations of episulfides utilize a two-step method, converting an olefin to an epoxide before its conversion to the episulfide using either thiocyanate or thiourea. Common uses of episulfides in both academic and industrial settings most often involve their use as monomers in polymerization reactions.
Preparation
A number of chemists in the early 1900s, including Staudinger and Pfenninger (1916), as well as Delepine (1920), were the first to prepare and report episulfides.[1] However, both of these accounts were isolated incidents on single substrates. It was not until 1934 that Dachlauer and Jackel devised the first general synthesis of episulfides from epoxides using alkali thiocyanates and thiourea, which is still one of the most common methods for preparing episulfides today.[1]
While the above method represents the most common method of preparation, episulfides can also be prepared from cyclic carbonates, hydroxy mercaptans, hydroxyalkyl halides, dihaloalkanes, and halo mercaptans, as well as a number of other preoxidized starting materials.[1] In addition, a reaction analogous to epoxidation has been reported involving the metal-catalyzed reaction of sulfur with alkenes.[2]
- alkene + "S" → episulfide
Reactions
Episulfides have an innate ring strain due to the nature of three-membered rings. Therefore, most reactions of episulfides involve the anionic nucleophilic opening of the heterocyclic ring.[1] However, the nucleophilic opening is typically only regioselective in the case of a terminal episulfide, in which case the nucleophilic attack heavily favors attack at the primary carbon over the secondary carbon. Nucleophiles that can be used include anionic hydride, sulfur, oxygen, nitrogen, and carbon.
In large part, episulfides are less common than epoxides simply due to the more common appearance of oxygen in natural products. However, episulfides have also been found to be much less stable than epoxides, limiting their use. This destabilization is due to the increased nucleophilic character of sulfur, which often leads to undesired polymerization reactions.
The few industrial processes involving episulfides take advantage of their extreme reactivity and mainly involve the polymerization of episulfide monomers. For instance, one process has been patented which describes the use of episulfide monomers to produce high-refractive-index plastic lenses.[3] While the exact polymerization reaction is unreported in the patent, a similar reaction involving the polymerization of episulfide monomers using [PPN]Cl/(salph)Cr(III)Cl as the catalyst has been reported.[4]
References
- Warren Chew; David N. Harpp (1993). "Recent aspects of thiirane chemistry". Journal of Sulfur Chemistry. 15 (1): 1–39. doi:10.1080/01961779308050628.
- 1 2 3 4 Sander, M. Thiiranes. Chem. Rev. 1966, 66(3), 297-339. doi:10.1021/cr60241a004
- ↑ Adam, W.; Bargon ,R. M.; Schenk, W. A. JACS 2003, 125, 3871-3876. doi:10.1021/ja029292p
- ↑ Ino, S. High-Refractive-Index Plastic Lenses Having Thermoplastic Films and Their Manufacture. JP Patent 2,010,066,648, March 25, 2010.
- ↑ Nakano, K.; Tatsumi, G.; Nazaki, K. (2007). "Synthesis of Sulfur-Rich Polymers: Copolymerization of Episulfide with Carbon Disulfide by Using [PPN]Cl/(salph)Cr(III)Cl System". J. Am. Chem. Soc. 129: 15116–15117. doi:10.1021/ja076056b.