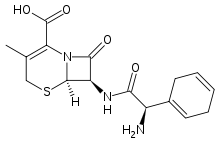
Cefradine
 | |
| Clinical data | |
|---|---|
| Trade names | Intracef, Velocef |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a601206 |
| Routes of administration | Oral, IM, IV |
| ATC code | J01DB09 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Well absorbed |
| Protein binding | <10% |
| Metabolism | Nil |
| Biological half-life | 0.9 hours |
| Excretion | Renal, unchanged |
| Identifiers | |
| |
| CAS Number |
38821-53-3 |
| PubChem (CID) | 38103 |
| IUPHAR/BPS | 4830 |
| DrugBank |
DB01333 |
| ChemSpider |
34933 |
| UNII |
9YA6SX5S4D |
| KEGG |
D00264 |
| ChEBI |
CHEBI:3547 |
| ChEMBL |
CHEMBL1604 |
| ECHA InfoCard | 100.049.199 |
| Chemical and physical data | |
| Formula | C16H19N3O4S |
| Molar mass | 349.406 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Cefradine (INN) (formerly cephradine BAN) is a first generation cephalosporin antibiotic.[1]
Indications
Cefradine has similar spectrum of activity to cefalexin. It is used in the following instances:
- Respiratory tract infections (such as tonsillitis, pharyngitis, and lobar pneumonia) caused by group A beta-hemolytic streptococci and S. pneumoniae (formerly D. pneumonia).[note 1]
- Otitis media caused by group A beta-hemolytic streptococci, S. pneumoniae (formerly D. pneumoniae), H. influenzae, and staphylococci.
- Skin and skin structure infections caused by staphylococci (penicillin-susceptible and penicillin-resistant) and beta-hemolytic streptococci.
- Urinary tract infections, including prostatitis, caused by E. coli, P. mirabilis, Klebsiella species, and enterococci (including Enterococcus faecalis). The high concentrations of cephradine achievable in the urinary tract will be effective against many strains of enterococci for which disc susceptibility studies indicate relative resistance.[note 2]
Formulations
Cefradine is distributed in the form of capsules containing 250 mg or 500 mg, as a syrup containing 250 mg/5 ml, or in vials for injection containing 500 mg or 1 g.
Production Names
The antibiotic is produced under a wide number of brand names across the world.[2]
- Bangladesh: Ancef (Unimed & Unihealth), Ancef forte (Unimed & Unihealth), Aphrin (Apex), Avlosef (ACI), Cefadin (Ziska), Cephadin (Square); Cephran and Cephran-DS (Opsonin), Cusef and Cusef DS (Delta), Dicef (Drug International), Dicef forte (Drug International), Dolocef (Techno), Efrad (Edruc), Extracef (Aristopharma), Extracef-DS (Aristopharma), Intracef (Beximco), Kefdrin (GlaxoSmithKline), Lebac (Square), Lebac Forte (Square), Medicef (Medicon), Mega-Cef (Hudson), Megacin (Novartis), Polycef (Renata), Procef (Incepta), Procef (Cefradine and Arginine) (Incepta), Procef forte (Incepta), Rocef and Rocef Forte DS (Healthcare), Sefin and Sefin DS (Orion), Sefnin (Monico), Sefrad and Sefrad DS (Sanofi-Aventis), Sefril and Sefril-DS (Acme), Sefro and Sefro-HS (Navana), Sephar and Sephar-DS (RAK), Septa (Doctor's Chemical Works), Sinaceph (Ibn Sina), SK-Cef and Sk-Cef DS (Eskayef), Supracef and Supracef-F (Bio-Pharma), Torped (Orion), Ultrasef (Jayson), Vecef and Vecef-DS (Asiatic Lab) and Velogen (General Pharma),Sinaceph(IBN Sina)
- China: Cefradine (Likang, Linheng Pharmaceutical, Livzon Zhuhai, Siyao Pharmaceuticals, WZ Pharm, China and Xinhua), Cephradine (New Asiatic Pharm and Sine Pharm), Kebili (Baiyunshan), Saifuding (The United Laboratories Ltd), Shen You (Shyndec), Taididing (Gosun), Velosef (Bristol-Myers Squibb), Xianyi (Dawnrays) and Xindadelei (Xincat)
- Columbia: Cefagram (Coaspharma), Cefrakov (Blaskov), Cefranil (Biotoscana), Cefrex (Labinco) and Kliacef (Bioquifar)
- Egypt: Cefadrin and Cefadrine (T3A), Cephradine (Eipico), Cephraforte (Sina), Farcosef (PBI)Fortecef (Meivo), Mepadrin (Mepaco), Ultracef (Misr) and Velosef (GlaxoSmithKline)
- France: Dexef (CSP, France)
- Hong Kong: Cefradine (Bright Future) and ChinaQualisef-250 (Quality Pharm)
- Indonesia: Dynacef (Dexa Medica), Velodine (Soho) and Velodrom (Ethica Industri Farmasi)
- Lebanon: Eskacef (Julphar, Lebanon), Julphacef (Julphar, Lebanon) and Velosef (Squibb Aebe)
- Lithuania: Tafril (Warszawskie Zaklady Farmaceutyczne Polfa)
- Myanmar: Sinaceph (IBN)
- Oman: Ceframed (Khimji Ramdas), Eskasef (Julphar), Omadine (NPI) and Velocef (Abeefe Bristol-Myers Squibb)
- Pakistan: Abidine (Alliance), Ada-Cef (Adamjee), Ag-cef (Saydon), Aksosef (Akson), Amspor (Wilshire), Anasef (Mass Pharma), Antimic (Danas), Atcosef (Atco), Bactocef (Swiss), Biocef (Searle), Velosef (Glaxo Smith Kline)
- Peru: Abiocef (Infermed), Cefradinal (Terbol, Peru), Cefradur (Atral), Cefrid (Infarmasa), Terbodina II (Terbol), Velocef (Abeefe Bristol-Myers Squibb), Velomicin (Siegfried)
- The Philippines: Altozef (Altomed), Racep (Samjin), Senadex (Nutramedica), Solphride (Lloyd),Yudinef (Biolink, Philippines), Zefadin (Singapore Pharmawealth Lifesciences), Zefradil (NCPC Hebei Huamin Pharma) and Zolicef (Harbin)
- Poland: Tafril (Polfa Tarchomin)
- Portugal: Cefalmin (Labesfal), Cefradur (Atral),
- South Africa: Cefril A (Bristol-Myers Squibb)
- South Korea: Cefradine (Hanmi) and Tricef (Kukj)
- Taiwan: Cefadin (Standard), Cefamid (Gentle), Cefin (Tai Yu) Cekodin (Swiss Pharm), Cephradine (Kojar and Root), Ceponin (Chi Sheng), Lacef (Tai Yu), Licef-A and Lisacef (Taiwan Biotech), Lofadine (Gentle), Recef (Purzer), S-60 (Yung Shin), Sefree (Panbiotic), Sephros (Yung Shin), Topcef (Gentle), Tydine (TTY Biopharm), Unifradine (Union) and U-Save (U-Liang)
- UK: Cefradune (Kent)
- Vietnam: Eurosefro (Navana Pharmaceuticals) and Incef (Inbionet)
Cefradine is known as Cefradina in Portuguse and Spanish and is produced by the following companies under this name: AC Farma, Peru; Andromaco, Chile; Anglopharma, Colombia; AZ Pharma, Colombia; Biogalenic, Venezuela; Bussié, Colombia; Elter - Medicamentos Genéricos, Venezuela; Farmindustria, Peru; Genfar, Colombia, Honduras and Peru; La Sante, Peru; La Santé, Colombia; Labesfal, Portugal; Lafrancol, Colombia; LCG, Peru; Marfan, Peru; Memphis, Colombia; Mintlab, Chile; MK, Colombia; Ophalac, Colombia; Procaps, Colombia and Vitalis, Colombia and Peru.
It is not approved by the FDA for use in the United States.
Synthesis
Noting that 1,4-cyclohexadiene rings are nearly as planar as benzene rings but of greatly different reactivity, a cephalosporin was synthesized with such a moiety.
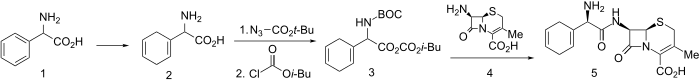
Birch reduction of D-α-phenylglycine led to diene (2). This was N-protected using tert-Butoxycarbonylazide and activated for amide formation via the mixed anhydride method using isobutylchloroformate to give 3. Mixed anhydride 3 reacted readily with 7-Aminodesacetoxycephalosporanic acid to give, after deblocking, cephadrine (5).
See also
- Cephapirin
- Cephacetrile
- Cefamandole
- Ampicillin (Has the same chemical formula)
Notes
- ↑ Penicillin is the usual drug of choice in the treatment and prevention of streptococcal infections, including the prophylaxis of rheumatic fever. Cefuroxime is generally effective in the eradication of streptococci from the nasopharynx
- ↑ Among beta-lactam antibiotics, ampicillin is the drug of choice for enterococcal urinary tract (E. faecalis) infection.
References
- ↑ British National Formulary (45 ed.). London: British Medical Association. 2003.
- ↑ {cite web|url=http://www.drugs.com/international/cefradine.html|title=Cefradine|access-date=5 May 2016}
- ↑ Dolfini, Joseph E.; Applegate, Harold E.; Bach, Georges; Basch, Harold; Bernstein, Jack; Schwartz, Joseph; Weisenborn, Frank L. (1971). "New class of semisynthetic penicillins and cephalosporins derived from D-2-(1,4-cyclohexadienyl)glycine". Journal of Medicinal Chemistry. 14 (2): 117. doi:10.1021/jm00284a008. PMID 5544394.