Calprotectin
Calprotectin is a complex of the mammalian proteins S100A8 and S100A9.[1][2] In the presence of calcium, calprotectin is capable of sequestering the essential nutrients manganese and zinc[2][3] via chelation.[4] This metal sequestration affords the complex antimicrobial properties.[2][3] Calprotectin is the only known antimicrobial manganese sequestration protein complex.[5] Calprotectin comprises as much as 60% of the soluble protein content of the cytosol of a neutrophil,[2][6][7] and it is secreted by an unknown mechanism during inflammation.[8] Faecal calprotectin has been used to detect intestinal inflammation (colitis or enteritis) and can serve as a biomarker for inflammatory bowel diseases.[6][9] Other names for calprotectin include MRP8-MRP14, calgranulin A and B, cystic fibrosis antigen, 60BB antigen, and 27E10 antigen.[2][8]
Structure

The human homologue of calprotectin is a 24 kDa dimer,[5] and is formed by the protein monomers S100A8 (10,835 Da) and S100A9 (13,242 Da).[10][11] The primary structure of calprotectin can vary between species. For instance, the mouse homologue of S100A8 is 10,295 Da,[12] while the S100A9 homologue is 13,049 Da.[13] Early size exclusion chromatography experiments incorrectly indicated that calprotectin had a molecular mass of 36.5 kDa;[2][7] occasionally this value is used in contemporary literature. Calprotectin S100A8-S100A9 dimers can non-covalently pair with one another to form 48 kDa tetramers.
Metal binding
Calprotectin has a high affinity for calcium, zinc and manganese.[6][7][14] Each of S100A8 and S100A9 contain two EF-hand type Ca2+ binding sites,[5][8] and calprotectin is able to bind a total of four calcium ions per dimer or eight calcium ions per tetramer.[15] Calcium binding induces a conformational change in the complex that improves its affinity for transition metals, and promotes tetramer formation.[2][5] A maximum of two transition metal ions may bind to each calprotectin S100A8-S100A9 dimer.[5]
A calprotectin dimer can bind only one manganese ion with high affinity, and it can do this only in the presence of calcium.[5][16] Zinc can bind at two sites within the calprotectin dimer, and this can occur in the absence of calcium.[2] Calcium, however, improves calprotectin's affinity for zinc.[5] While calprotectin metal binding occurs at the interface of S100A9 and S100A8 monomers, the independent monomers have some capacity for zinc binding, and may contribute to zinc homeostasis within mammals.[2][10][11]
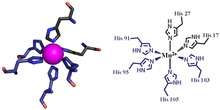
The first of the two calprotectin metal binding sites consists of a His3Asp motif, with S100A8 contributing two histidine ligands (His83 and His87), and S100A9 contributing a histidine and an aspartic acid ligand (His20 and Asp30).[5] The second site can coordinate metals through a tetra-histidine (His4) or a hexa-histidine (His6) binding motif. In the case of His4 binding, S100A8 coordinates through both His17 and His27 while S100A9 coordinates through His91 and His95.[5] In hexa-histidine binding two further histidine residues, His103 and His105, are recruited from the C-terminal end of S100A9 to enable octahedral coordination of the transition metal.[5] Manganese is bound by the calprotectin dimer at this His6 site.[5] Zinc can be bound to either of the sites that form at the interface between S100A8 and S100A9 monomers.[5][16]
Antimicrobial properties
Transition metals are essential to the survival of all organisms.[17] Mammals strictly limit metal availability as a part of the innate immune system, and this helps prevent infection by microbes and fungi.[17] Calprotectin was first described in the 1980s as a mammalian antimicrobial protein that acts through the sequestration of zinc.[1][2][5] It is now known that calprotectin also has antibacterial and antifungal properties that arise from its ability to sequester manganese.[3][5] Calprotectin is the only known antimicrobial agent that acts through manganese sequestration.[5]
Calprotectin constitutes up to 60% of soluble protein content in the cytosol of neutrophil granulocytes,[2][6][7] and it can be found at a lower concentration in monocytes, macrophages, and squamous epithelial cells.[2][6][7] Calprotectin enters into pus and abscess fluid during neutrophil cell death, along with other antimicrobial proteins.[2]
Mammalian cells secrete calprotectin during the inflammatory response. For instance, calprotectin is secreted in the mouth during inflammation of the gingiva and during oral candidiasis infection.[18][19] People who have mutations in the calprotectin gene appear susceptible to serious gum infections.[18] Manganese sequestration by calprotectin is likely important during lung inflammation.[3] The exact mechanism by which S100A8 and S100A9 is secreted by mammalian cells during inflammation remains unknown.[8]
Faecal calprotectin
Calprotectin becomes available in the intestinal lumen via leukocyte shedding,[1] active secretion,[2][7] cell disturbance, and cell death.[1][7] This results in elevated faecal calprotectin levels, which can be detected in the stool.[1][7] Elevated faecal calprotectin levels therefore indicate migration of neutrophils into the intestinal mucosa, which occurs during intestinal inflammation.[1][7][14] As people with active inflammatory bowel diseases (IBD) such as ulcerative colitis or Crohn disease have as much as a 10-fold increase in faecal calprotectin levels,[6] the measurement of faecal calprotectin can serve as a biochemical test for these diseases.
Although a relatively new test, faecal calprotectin is regularly used as an indicator for IBD during treatment, and as a diagnostic marker.[9] Faecal calprotectin tests can also function in distinguishing patients with irritable bowel syndrome from those with IBD.[1][7] Calprotectin is useful as a marker, as it is resistant to enzymatic degradation, and can be easily measured in faeces.[20] Although faecal calprotectin correlates significantly with disease activity in people with confirmed IBD,[21] elevated faecal calprotectin can be a false-positive indicator of IBD under some conditions. Importantly, intake of proton pump inhibitor is associated with significantly elevated calprotectin values.[22] Furthermore, positive faecal calprotectin does not help in localizing IBD, or in distinguishing ulcerated colitis from Crohn disease.[1] Faecal calprotectin can also indicate other gastrointestinal conditions such as colorectal cancer, gastroenteritis, and food intolerance.[1] Calprotectin levels vary depending on age, comorbidity, and may vary day-to-day within individuals.[1] Faecal calprotectin could be used as a preliminary screen in otherwise functional patients suspected of having IBD, or as a means of following mucosal healing.[1] The potential for using faecal calprotectin in this way is debated, however, and cut-off levels have not been agreed upon.[1]
See also
References
- 1 2 3 4 5 6 7 8 9 10 11 12 Lehmann, F. S.; Burri, E.; Beglinger, C. (13 October 2014). "The role and utility of faecal markers in inflammatory bowel disease". Therapeutic Advances in Gastroenterology. 8 (1): 23–36. doi:10.1177/1756283X14553384. PMC 4265086
 . PMID 25553077.
. PMID 25553077. - 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Striz, I; Trebichavsky, I (2004). "Calprotectin - a pleiotropic molecule in acute and chronic inflammation.". Physiological research / Academia Scientiarum Bohemoslovaca. 53 (3): 245–53. PMID 15209531.
- 1 2 3 4 Costa, Lucio G; Aschner, Michael (2014). Manganese in Health and Disease. Royal Society of Chemistry. p. 146. ISBN 1849739439. Retrieved 27 January 2015.
- ↑ Clark, HL; et al. (2016), "Zinc and manganese chelation by neutrophil s100a8/a9 (calprotectin) limits extracellular Aspergillus fumigatus hyphal growth and corneal infection", J Immunol, 196 (1): 336–344, doi:10.4049/jimmunol.1502037, PMID 26582948.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Brophy, Megan Brunjes; Nolan, Elizabeth M. (16 January 2015). "Manganese and Microbial Pathogenesis: Sequestration by the Mammalian Immune System and Utilization by Microorganisms". ACS Chemical Biology. 10: 150116125412006. doi:10.1021/cb500792b.
- 1 2 3 4 5 6 Marshall, William Marshall,; Lapsley, Marta; Day, Andrew; Ayling, Ruth (2014). Clinical Biochemistry: Metabolic and Clinical Aspects (3 ed.). Elsevier Health Sciences, 2014. ISBN 9780702054785. Retrieved 19 January 2015.
- 1 2 3 4 5 6 7 8 9 10 Gupta, Ramesh (2014). Biomarkers in toxicology. San Diego, CA: Academic Press. pp. 272–273. ISBN 9780124046498. Retrieved 19 January 2015.
- 1 2 3 4 Celio, Marco R.; Pauls, Thomas; Schwaller, Beat (1996). Guidebook to the calcium-binding proteins. Oxford: Sambrook & Tooze Publication at Oxford University Press. pp. 147–148. ISBN 0198599501.
- 1 2 van Rheenen PF, Van de Vijver E, Fidler V (2010). "Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis". BMJ. 341: c3369. doi:10.1136/bmj.c3369. PMC 2904879
 . PMID 20634346. Lay summary – MedScape.
. PMID 20634346. Lay summary – MedScape. - 1 2 UniProt Consortium. "P05109- S10A8_HUMAN". http://www.uniprot.org/. UniProt Consortium. Retrieved 21 January 2015. External link in
|website=(help) - 1 2 UniProt Consortium. "P06702- S10A9_HUMAN". http://www.uniprot.org/. UniProt Consortium. Retrieved 21 January 2015. External link in
|website=(help) - ↑ UniProt Consortium. "P27005- S10A8_MOUSE". http://www.uniprot.org/. UniProt Consortium. Retrieved 21 January 2015. External link in
|website=(help) - ↑ UniProt Consortium. "P31725- S10A9_MOUSE". http://www.uniprot.org/. UniProt Consortium. Retrieved 21 January 2015. External link in
|website=(help) - 1 2 Evans, G.O. (2009). Animal Clinical Chemistry: A Practical Handbook for Toxicologists and Biomedical Researchers (2 ed.). Boca Raton: Taylor & Francis. pp. 107–108. ISBN 9781420080124. Retrieved 19 January 2015.
- ↑ Strupat, K; Rogniaux, H; Van Dorsselaer, A; Roth, J; Vogl, T (September 2000). "Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 are confirmed by electrospray ionization-mass analysis.". Journal of the American Society for Mass Spectrometry. 11 (9): 780–8. doi:10.1016/s1044-0305(00)00150-1. PMID 10976885.
- 1 2 Maret, Wolfgang; Wedd, Anthony (2014). Binding, transport and storage of metal ions in biological cells. [S.l.]: Royal Soc Of Chemistry. p. 271. ISBN 9781849735995. Retrieved 27 January 2015.
- 1 2 Hood, M; Skaar, E (2013). "Nutritional immunity: transition metals at the pathogen-host interface". Nature Reviews Microbiology. 10 (8): 525–537. doi:10.1038/nrmicro2836. PMC 3875331
 . PMID 22796883.
. PMID 22796883. - 1 2 Schaechter, Moselio (2009). Encyclopedia of microbiology (3 ed.). [S.l.]: Elsevier. p. 570. ISBN 0123739446. Retrieved 27 January 2015.
- ↑ Vacharaksa, Anjalee (2007). Restricted HIV-1 Infection Increases Susceptibility of Candida Infection in Oral Keratinocytes. ProQuest. p. 20. ISBN 9780549367666. Retrieved 27 January 2015.
- ↑ Tibble J, Teahon K, Thjodleifsson B, Roseth A, Sigthorsson G, Bridger S, Foster R, Sherwood R, Fagerhol M, Bjarnason I (2000). "A simple method for assessing intestinal inflammation in Crohn's disease". Gut. 47 (4): 506–13. doi:10.1136/gut.47.4.506. PMC 1728060
 . PMID 10986210.
. PMID 10986210. - ↑ D'Haens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, Geens P, Iwens D, Aerden I, Van Assche G, Van Olmen G, Rutgeerts P (2012). "Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease". Inflamm. Bowel Dis. 18 (12): 2218–24. doi:10.1002/ibd.22917. PMID 22344983.
- ↑ Poullis A, Foster R, Mendall MA, Shreeve D, Wiener K (2003). "Proton pump inhibitors are associated with elevation of faecal calprotectin and may affect specificity". Eur J Gastroenterol Hepatol. 15 (5): 573–4; author reply 574. doi:10.1097/00042737-200305000-00021. PMID 12702920.