Clostridium difficile infection
| Clostridium difficile infection | |
|---|---|
| Synonyms | pseudomembranous colitis, C. difficile associated diarrhea (CDAD), Clostridium difficile colitis |
|
| |
| Pathological specimen showing pseudomembranous colitis | |
| Classification and external resources | |
| Specialty | Infectious disease |
| ICD-10 | A04.7 |
| ICD-9-CM | 008.45 |
| MedlinePlus | 000259 |
| eMedicine | med/1942 |
| MeSH | D004761 |
Clostridium difficile infection (CDI) is a symptomatic infection due to the spore-forming bacterium, Clostridium difficile.[1] Symptoms include watery diarrhea, fever, nausea, and abdominal pain. It makes up about 20% of cases of antibiotic-associated diarrhea. Complications may include pseudomembranous colitis, toxic megacolon, perforation of the colon, and sepsis.[2]
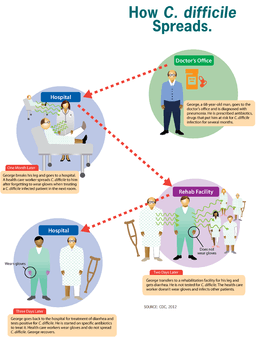
Clostridium difficile infection is spread by bacterial spores found within feces. Surfaces may become contaminated with the spores with further spread occurring via the hands of healthcare workers. Risk factors for infection include antibiotic or proton pump inhibitors use, hospitalization, other health problems, and older age. Diagnosis is by stool culture or testing for the bacteria's DNA or toxins. If a person tests positive but has no symptoms it is known as C. difficile colonization rather than an infection.[2]
Prevention is by limiting antibiotic use, hand washing, and terminal room cleaning in hospital.[1] Discontinuation of antibiotics may result in resolution of symptoms within three days in about 20% of those infected. Often the antibiotics metronidazole, vancomycin or fidaxomicin will cure the infection. Retesting after treatment, as long as the symptoms have resolved, is not recommended as the person may remain colonized.[2] Recurrence have been reported in up to 25% of people.[3] There is tentative evidence that fecal microbiota transplantation and probiotics may decrease the risk of recurrence.[1]
C. difficile infections occur in all areas of the world.[4] About 453,000 cases occurred in the United States in 2011 resulting in 29,000 deaths.[1][5] Rates of disease globally have increased between 2001 and 2016.[1][4] Women are more often affected than men.[1] The bacteria was discovered in 1935 and found to be disease causing in 1978.[4] In the United States healthcare acquired infections increase cost of care by 1.5 billion USD each year.[6]
Signs and symptoms
Signs and symptoms of CDI range from mild diarrhea to severe life-threatening inflammation of the colon.[7]
In adults, a clinical prediction rule found the best signs to be significant diarrhea ("new onset of more than three partially formed or watery stools per 24-hour period"), recent antibiotic exposure, abdominal pain, fever (up to 40.5 °C or 105 °F), and a distinctive foul odor to the stool resembling horse manure.[8] In a population of hospitalized patients, prior antibiotic treatment plus diarrhea or abdominal pain had a sensitivity of 86% and a specificity of 45%.[9] In this study with a prevalence of positive cytotoxin assays of 14%, the positive predictive value was 18% and the negative predictive value was 94%.
In children, the most prevalent symptom of a CDI is watery diarrhea with at least three bowel movements a day for two or more days, which may be accompanied by fever, loss of appetite, nausea, and/or abdominal pain.[10] Those with a severe infection also may develop serious inflammation of the colon and have little or no diarrhea.
Cause
Infection with C. difficile bacteria is responsible for C. difficile diarrhea.
C. difficile

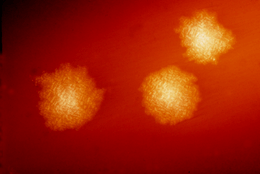
Clostridia are anaerobic motile bacteria, ubiquitous in nature, and especially prevalent in soil. Under the microscope, they appear as long, irregular (often drumstick- or spindle-shaped) cells with a bulge at their terminal ends. Under Gram staining, C. difficile cells are Gram-positive and show optimum growth on blood agar at human body temperatures in the absence of oxygen. When stressed, the bacteria produce spores that are able to tolerate extreme conditions that the active bacteria cannot tolerate.[11]
C. difficile may become established in the human colon; it is present in 2–5% of the adult population.[11]
Pathogenic C. difficile strains produce multiple toxins.[12] The most well-characterized are enterotoxin (Clostridium difficile toxin A) and cytotoxin (Clostridium difficile toxin B), both of which may produce diarrhea and inflammation in infected patients, although their relative contributions have been debated.[11] Toxins A and B are glucosyltransferases that target and inactivate the Rho family of GTPases. Toxin B (cytotoxin) induces actin depolymerization by a mechanism correlated with a decrease in the ADP-ribosylation of the low molecular mass GTP-binding Rho proteins.[13] Another toxin, binary toxin, also has been described, but its role in disease is not fully understood.[14]
Antibiotic treatment of CDIs may be difficult, due both to antibiotic resistance and physiological factors of the bacteria (spore formation, protective effects of the pseudomembrane).[11] The emergence of a new and highly toxic strain of C. difficile that is resistant to fluoroquinolone antibiotics such as ciprofloxacin and levofloxacin, said to be causing geographically dispersed outbreaks in North America, was reported in 2005.[15] The U.S. Centers for Disease Control and Prevention in Atlanta warned of the emergence of an epidemic strain with increased virulence, antibiotic resistance, or both.[16]
C. difficile is transmitted from person to person by the fecal-oral route. The organism forms heat-resistant spores that are not killed by alcohol-based hand cleansers or routine surface cleaning. Thus, these spores survive in clinical environments for long periods. Because of this, the bacteria may be cultured from almost any surface. Once spores are ingested, their acid-resistance allows them to pass through the stomach unscathed. Upon exposure to bile acids, they germinate and multiply into vegetative cells in the colon.
In 2005, molecular analysis led to the identification of the C. difficile strain type characterized as group BI by restriction endonuclease analysis, as North American pulse-field-type NAP1 by pulsed-field gel electrophoresis and as ribotype 027; the differing terminology reflects the predominant techniques used for epidemiological typing. This strain is referred to as C. difficile BI/NAP1/027.[17]
Risk factors
Antibiotics
C. difficile colitis is associated most strongly with the use of the following antibiotics: fluoroquinolones, cephalosporins, and clindamycin.[18]
Some research suggests the routine use of antibiotics in the raising of livestock is contributing to outbreaks of bacterial infections such as C. difficile.[19]
Healthcare environment
People are most often infected in hospitals, nursing homes, or other medical institutions, although infection outside medical settings is increasing. The rate of C. difficile acquisition is estimated to be 13% in patients with hospital stays of up to two weeks, and 50% with stays longer than four weeks.[20]
Long-term hospitalization or residence in a nursing home within the previous year are independent risk factors for increased colonization.[21]
Acid suppression medication
Increasing rates of community-acquired CDI are associated with the use of medication to suppress gastric acid production: H2-receptor antagonists increased the risk 1.5-fold, and proton pump inhibitors by 1.7 with once-daily use and 2.4 with more than once-daily use.[22][23]
Pathophysiology
The use of systemic antibiotics, including (but not limited to) any penicillin-based antibiotic such as ampicillin, cephalosporins, and clindamycin, causes the normal bacterial flora of the bowel to be altered. In particular, when the antibiotic kills off other competing bacteria in the intestine, any bacteria remaining will have less competition for space and nutrients. The net effect is to permit more extensive growth than normal of certain bacteria. Clostridium difficile is one such type of bacterium. In addition to proliferating in the bowel, C. difficile also produces toxins. Without either toxin A or toxin B, C. difficile may colonize the gut, but is unlikely to cause pseudomembranous colitis.[24] The colitis associated with severe infection is part of an inflammatory reaction, with the "pseudomembrane" formed by a viscous collection of inflammatory cells, fibrin, and necrotic cells.[11]
Diagnosis
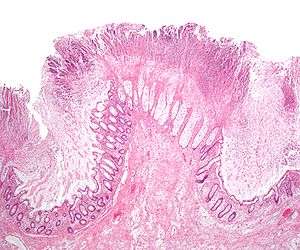
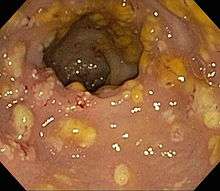
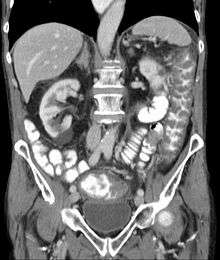
Prior to the advent of tests to detect C. difficile toxins, the diagnosis most often was made by colonoscopy or sigmoidoscopy. The appearance of "pseudomembranes" on the mucosa of the colon or rectum is highly suggestive, but not diagnostic of the condition.[25] The pseudomembranes are composed of an exudate made of inflammatory debris, white blood cells. Although colonoscopy and sigmoidoscopy are still employed, now stool testing for the presence of C. difficile toxins is frequently the first-line diagnostic approach. Usually, only two toxins are tested for—toxin A and toxin B—but the organism produces several others. This test is not 100% accurate, with a considerable false-negative rate even with repeat testing.
Cytotoxicity assay
C. difficile toxins have a cytopathic effect in cell culture, and neutralization of any effect observed with specific antisera is the practical gold standard for studies investigating new CDI diagnostic techniques.[11] Toxigenic culture, in which organisms are cultured on selective media and tested for toxin production, remains the gold standard and is the most sensitive and specific test, although it is slow and labor-intensive.[26]
Toxin ELISA
Assessment of the A and B toxins by enzyme-linked immunosorbent assay (ELISA) for toxin A or B (or both) has a sensitivity of 63–99% and a specificity of 93–100%.
Previously, experts recommended sending as many as three stool samples to rule out disease if initial tests are negative, however, evidence suggests repeated testing during the same episode of diarrhea is of limited value and should be discouraged.[27] C. difficile toxin should clear from the stool of previously infected patients if treatment is effective. Many hospitals only test for the prevalent toxin A. Strains that express only the B toxin are now present in many hospitals, however, so testing for both toxins should occur.[28][29] Not testing for both may contribute to a delay in obtaining laboratory results, which is often the cause of prolonged illness and poor outcomes.
Other stool tests
Stool leukocyte measurements and stool lactoferrin levels also have been proposed as diagnostic tests, but may have limited diagnostic accuracy.[30]
PCR
Testing of stool samples by real-time polymerase chain reaction is able to pick up the disease about 90% of the time and when positive is incorrectly positive about 4% of the time.[31] Multistep PCR testing algorithms can improve overall performance. Repeat testing may be misleading, and testing specimens more than once every seven days in patients without new symptoms is highly unlikely to yield useful information.[32]
Prevention
Antibiotics
The most effective method for preventing CDI is proper antimicrobial prescribing. In the hospital setting, where CDI is most common, nearly all patients who develop CDI are exposed to antimicrobials. Although proper antimicrobial prescribing sounds easy to do, about 50% of antimicrobial use is considered inappropriate. This is consistent whether in the hospital, clinic, community, or academic setting. A decrease in CDI by limiting antibiotics or by limiting unnecessary antimicrobial prescriptions in general, both in an outbreak and non-outbreak setting has been demonstrated to be most strongly associated with reduced CDI. Further, reactions to medication may be severe: CDI infections were the most common contributor to adverse drug events seen in U.S. hospitals in 2011.[33]
Probiotics
Some evidence indicates probiotics may be useful to prevent infection and recurrence.[34][35] Treatment with Saccharomyces boulardii in those who are not immunocompromised with C. difficile also may be useful.[36][37] Initially, in 2010, the Infectious Diseases Society of America recommended against their use due to the risk of complications.[34][36] Subsequent reviews, however, did not find an increase in adverse effects with treatment,[35] and overall treatment appears safe.[38]
Infection control
Rigorous infection protocols are required to minimize this risk of transmission.[39] Infection control measures, such as wearing gloves and noncritical medical devices used for a single person with CDI, are effective at prevention.[40] This works by limiting the spread of C. difficile in the hospital setting. In addition, washing with soap and water will eliminate the spores from contaminated hands, but alcohol-based hand rubs are ineffective.[41]
Bleach wipes containing 0.55% sodium hypochlorite have been shown to kill the spores and prevent transmission between patients.[42] Installing lidded toilets and closing the lid prior to flushing also reduces the risk of contamination.[43]
Those who have CDIs should be in rooms with other people with CDIs or by themselves when in hospital.[40]
Common hospital disinfectants are ineffective against C. difficile spores and, in fact, may promote spore formation, however, disinfectants containing a 10:1 ratio of water to bleach effectively kill the spores.[44] Hydrogen peroxide vapor (HPV) systems used to sterilize a patient room post-discharge have been shown to reduce infection rates and to reduce risk of infection to subsequent patients. The incidence of CDI was reduced by 53%[45] or 42%[46] through use of HPV. Ultraviolet cleaning devices and housekeeping staff especially dedicated to disinfecting the rooms of patients infected with C. difficile after discharge may be effective.[47]
Treatment
Carrying C. difficile without symptoms is common. Treatment in those without symptoms is controversial. In general, mild cases do not require specific treatment.[11][48] Oral rehydration therapy is useful in treating dehydration associated with the diarrhea.
Medications
A number of different antibiotics are used for C. difficile, with the available agents being more or less equally effective.[49]
- Metronidazole typically is the initial drug of choice for mild to moderate disease, because of lower price.[36] Typically it is taken three times a day for 10 days.[50]
- Oral vancomycin is preferred for severe disease.[36] Additionally, vancomycin may be used to treat mild-to-moderate disease if diarrhea persists after a course of metronidazole.[50] Since metronidazole has the potential to cause birth defects, pregnant women with Clostridium difficile infection may be treated with vancomycin regardless of disease severity.[50] Vancomycin and metronidazole, however, appear to be equally effective.[48] Typical vancomycin dosage is taken four times daily for 10 days.[50] Vancomyin may be given rectally if the person develops an ileus and cannot take medications by mouth.[51]
- Fidaxomicin has been found to be as effective as vancomycin in those with mild to moderate disease.[52] It is tolerated as well as vancomycin,[53] and may have a lower risk of recurrence.[49] It may be used in those who have recurrent infections and have not responded to other antibiotics.[52]
Drugs used to slow or stop diarrhea such as loperamide may worsen C. difficile disease, so are not recommended.[54] Cholestyramine, an ion exchange resin, is effective in binding both toxin A and B, slowing bowel motility, and helping prevent dehydration.[55] Cholestyramine is recommended with vancomycin. A last-resort treatment in those who are immunosuppressed is intravenous immunoglobulin (IVIG).[55]
Probiotics
Evidence to support the use of probiotics in the treatment of active disease is insufficient.[36][56] Thus in this situation, they are recommended neither as an add-on to standard therapy nor for use alone.[57]
Stool transplant
Fecal bacteriotherapy, also known as a stool transplant, is approximately 85% to 90% effective in those for whom antibiotics have not worked.[58][59] It involves infusion of bacterial flora acquired from the feces of a healthy donor to reverse the bacterial imbalance responsible for the recurring nature of the infection.[60] The procedure replaces normal, healthy colonic flora that had been wiped out by antibiotics, and reestablishes resistance to colonization by Clostridium difficile.[61] Side effects, at least initially, are few.[59]
There is evidence that looks hopeful that fecal transplant can be delivered in the form of a pill.[62] They are available in the United States but are not FDA-approved as of 2015.[63]
Surgery
In those with severe C. difficile colitis, colectomy may improve the outcomes.[64] Specific criteria may be used to determine who will benefit most from surgery.[65]
Prognosis
After a first treatment with metronidazole or vancomycin, C. difficile recurs in about 20% of people. This increases to 40% and 60% with subsequent recurrences.[66]
Epidemiology
C. difficile diarrhea is estimated to occur in 8 out of 100,000 people each year.[67] Among those who are admitted to hospital, it occurs in between 4 and 8 people per 1,000.[67] In 2011 it resulted in about half a million infections and 29,000 deaths in the United States.[5] Due in part to the emergence of a fluoroquinolone resistant strain, C. difficile-related deaths increased 400% between the years 2000 and 2007 in the United States.[68]
History
Initially named Bacillus difficilis by Hall and O'Toole in 1935 because it was resistant to early attempts at isolation and grew very slowly in culture, it was renamed in 1970.[66][69]
Pseudomembranous colitis first was described as a complication of C. difficile infection in 1978,[70] when a toxin was isolated from patients suffering from pseudomembranous colitis and Koch's postulates were met.
Notable outbreaks
- On 4 June 2003, two outbreaks of a highly virulent strain of this bacterium were reported in Montreal, Quebec, and Calgary, Alberta. Sources put the death count to as low as 36 and as high as 89, with approximately 1,400 cases in 2003 and within the first few months of 2004. CDIs continued to be a problem in the Quebec healthcare system in late 2004. As of March 2005, it had spread into the Toronto area, hospitalizing ten people. One died while the others were being discharged.
- A similar outbreak took place at Stoke Mandeville Hospital in the United Kingdom between 2003 and 2005. The local epidemiology of C. difficile may offer clues on how its spread may relate to the time a patient spends in hospital and/or a rehabilitation center. It also samples the ability of institutions to detect increased rates, and their capacity to respond with more aggressive hand-washing campaigns, quarantine methods, and the availability of yogurt containing live cultures to patients at risk for infection.
- Both the Canadian and English outbreaks possibly were related to the seemingly more virulent strain NAP1/027 of the bacterium. Known as Quebec strain, it has been implicated in an epidemic at two Dutch hospitals (Harderwijk and Amersfoort, both 2005). A theory for explaining the increased virulence of 027 is that it is a hyper producer of both toxins A and B and that certain antibiotics may stimulate the bacteria to hyperproduce.
- On 1 October 2006, C. difficile was said to have killed at least 49 people at hospitals in Leicester, England, over eight months, according to a National Health Service investigation. Another 29 similar cases were investigated by coroners.[71] A UK Department of Health memo leaked shortly afterward revealed significant concern in government about the bacterium, described as being "endemic throughout the health service"[72]
- On 27 October 2006, nine deaths were attributed to the bacterium in Quebec.[73]
- On 18 November 2006, the bacterium was reported to have been responsible for twelve deaths in Quebec. This twelfth reported death was only two days after the St. Hyacinthe's Honoré Mercier announced the outbreak was under control. Thirty-one patients were diagnosed with CDIs. Cleaning crews took measures in an attempt to clear the outbreak.[74]
- C. difficile was mentioned on 6,480 death certificates in 2006 in UK.[75]
- On 27 February 2007, a new outbreak was identified at Trillium Health Centre in Mississauga, Ontario, where 14 people were diagnosed with CDIs. The bacteria were of the same strain as the one in Quebec. Officials have not been able to determine whether C. difficile was responsible for deaths of four patients over the prior two months.[76]
- Between February and June 2007, three patients at Loughlinstown Hospital in Dublin, Ireland, were found by the coroner to have died as a result of C. difficile infection. In an inquest, the Coroner's Court found the hospital had no designated infection control team or consultant microbiologist on staff.[77]
- Between June 2007 and August 2008, Northern Health and Social Care Trust Northern Ireland, Anrtim Area, Braid Valley, Mid Ulster Hospitals were the subject of inquiry. During the inquiry, expert reviewers concluded that C. difficile was implicated in 31 of these deaths, as the underlying cause in 15, and as a contributory cause in 16. During that time, the review also noted 375 instances of CDIs in patients.[78]
- In October 2007, Maidstone and Tunbridge Wells NHS Trust was heavily criticized by the Healthcare Commission regarding its handling of a major outbreak of C. difficile in its hospitals in Kent from April 2004 to September 2006. In its report, the Commission estimated approximately 90 patients "definitely or probably" died as a result of the infection.[79][80]
- In November 2007, the 027 strain spread into several hospitals in southern Finland, with ten deaths out of 115 infected patients reported on 2007-12-14.[81]
- In November 2009, four deaths at Our Lady of Lourdes Hospital in Ireland have possible links to CDI. A further 12 patients tested positive for infection, and another 20 showed signs of infection.[82]
- From February 2009 to February 2010, 199 patients at Herlev hospital in Denmark were suspected of being infected with the 027 strain. In the first half of 2009, 29 died in hospitals in Copenhagen after they were infected with the bacterium.[83]
- In May 2010, a total of 138 patients at four different hospitals in Denmark were infected with the 027 strain [84] plus there were some isolated occurrences at other hospitals.[85]
- In May 2010, there were 14 fatalities related to the bacterium in the Australian state of Victoria. Two years later, the same strain of the bacterium was detected in New Zealand.[86]
- On 28 May 2011, an outbreak in Ontario had been reported, with 26 fatalities as of 24 July 2011.[87]
- In 2012/2013, a total of 27 people at one hospital in the south of Sweden (Ystad) were infected with 10 deaths. 5 died of the strain 017.[88]
Pronunciation
The anglicized pronunciation /klɒsˈtrɪdiəm dᵻˈfɪsᵻliː/ is common, though a more classical /dᵻˈfɪkᵻleɪ/ is also used. The classical Latin sound is /dɨˈffɪkɨle/. Difficile commonly is mispronounced /diːfiˈsiːl/, as though it were French. The word is from the Greek kloster (κλωστήρ), "spindle",[89] and Latin difficile, "difficult, obstinate".[90]
Research
- Efforts to generate a vaccine are ongoing as of 2015 with promising initial results.[4]
- CDA-1 and CDB-1 (also known as MDX-066/MDX-1388 and MBL-CDA1/MBL-CDB1) is an investigational, monoclonal antibody combination co-developed by Medarex and Massachusetts Biologic Laboratories (MBL) to target and neutralize C. difficile toxins A and B, for the treatment of CDI. Merck & Co., Inc. gained worldwide rights to develop and commercialize CDA-1 and CDB-1 through an exclusive license agreement signed in April 2009. It is intended as an add-on therapy to one of the existing antibiotics to treat CDI.[91][92][93]
- Nitazoxanide is a synthetic nitrothiazolyl-salicylamide derivative indicated as an antiprotozoal agent (FDA-approved for the treatment of infectious diarrhea caused by Cryptosporidium parvum and Giardia lamblia) and also is currently being studied in C. difficile infections vs. vancomycin.[94]
- Rifaximin,[94] is a clinical-stage semisynthetic, rifamycin-based nonsystemic antibiotic for CDI. It is FDA-approved for the treatment of infectious diarrhea and is being developed by Salix Pharmaceuticals.
- Other drugs for the treatment of CDI are under development and include rifalazil,[94] tigecycline,[94] ramoplanin,[94] ridinilazole, and SQ641.[95]
- Research has studied whether the vermiform appendix has any importance in, C. difficile. The appendix is thought to have a function of housing good gut flora. In a study conducted in 2011, it was shown that when C. difficile bacteria were introduced into the gut, the appendix housed cells that increased the antibody response of the body. The B cells of the appendix migrate, mature, and increase the production of toxin A-specific IgA and IgG antibodies, leading to an increased probability of good gut flora surviving against the C. difficile bacteria.[96]
- Taking non toxic types of C. difficile after an infection has promising results with respect to preventing future infections.[97]
- Bezlotoxumab, a human monoclonal antibody, was approved for the treatment of C. Difficile by the FDA in 2016. It is given by injection. It is hoped that the medication will become available in the first quarter of 2017.[98]
Other animals
- Colitis-X (in horses)
References
- 1 2 3 4 5 6 Butler, M; Olson, A; Drekonja, D; Shaukat, A; Schwehr, N; Shippee, N; Wilt, TJ (March 2016). "Early Diagnosis, Prevention, and Treatment of Clostridium difficile: Update". AHRQ Comparative Effectiveness Reviews.: vi,1. PMID 27148613.
- 1 2 3 "Frequently Asked Questions about Clostridium difficile for Healthcare Providers". CDC. March 6, 2012. Retrieved 5 September 2016.
- ↑ Long, Sarah S.; Pickering, Larry K.; Prober, Charles G. (2012). Principles and Practice of Pediatric Infectious Diseases (4 ed.). Elsevier Health Sciences. p. 979. ISBN 1455739855.
- 1 2 3 4 Lessa, FC; Gould, CV; McDonald, LC (August 2012). "Current status of Clostridium difficile infection epidemiology.". Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 55 Suppl 2: S65–70. PMID 22752867.
- 1 2 Lessa, Fernanda C.; Mu, Yi; Bamberg, Wendy M.; Beldavs, Zintars G.; Dumyati, Ghinwa K.; Dunn, John R.; Farley, Monica M.; Holzbauer, Stacy M.; Meek, James I.; Phipps, Erin C.; Wilson, Lucy E.; Winston, Lisa G.; Cohen, Jessica A.; Limbago, Brandi M.; Fridkin, Scott K.; Gerding, Dale N.; McDonald, L. Clifford (26 February 2015). "Burden of Infection in the United States". New England Journal of Medicine. 372 (9): 825–834. doi:10.1056/NEJMoa1408913. PMID 25714160.
- ↑ Leffler, DA; Lamont, JT (16 April 2015). "Clostridium difficile infection.". The New England journal of medicine. 372 (16): 1539–48. PMID 25875259.
- ↑ Joshi NM, Macken L, Rampton D (2012). "Inpatient diarrhoea and Clostridium difficile infection". Clinical Medicine. 12 (6): 583–588. doi:10.7861/clinmedicine.12-6-583.
- ↑ Bomers, Marije (April 2015). "Rapid, Accurate, and On-Site Detection of C. difficile in Stool Samples". The American Journal of Gastroenterology. 110 (4): 588–594. doi:10.1038/ajg.2015.90. PMID 25823766.
- ↑ Katz DA, Lynch ME, Littenberg B (May 1996). "Clinical prediction rules to optimize cytotoxin testing for Clostridium difficile in hospitalized patients with diarrhea". The American Journal of Medicine. 100 (5): 487–95. doi:10.1016/S0002-9343(95)00016-X. PMID 8644759.
- ↑ Moreno MA, Furtner F, Rivara FP (June 2013). "Clostridium difficile: A Cause of Diarrhea in Children". JAMA Pediatrics. 167 (6): 592. doi:10.1001/jamapediatrics.2013.2551. PMID 23733223.
- 1 2 3 4 5 6 7 Ryan KJ, Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. pp. 322–4. ISBN 0-8385-8529-9.
- ↑ Di Bella, Stefano; Ascenzi, Paolo; Siarakas, Steven; Petrosillo, Nicola; di Masi, Alessandra (2016-01-01). "Clostridium difficile Toxins A and B: Insights into Pathogenic Properties and Extraintestinal Effects". Toxins. 8 (5). doi:10.3390/toxins8050134. ISSN 2072-6651. PMC 4885049
 . PMID 27153087.
. PMID 27153087. - ↑ Just I, Selzer J, von Eichel-Streiber C, Aktories K (1995). "The low molecular mass GTP-binding protein Rh is affected by toxin a from Clostridium difficile". The Journal of Clinical Investigation. 95 (3): 1026–31. doi:10.1172/JCI117747. PMC 441436
 . PMID 7883950.
. PMID 7883950. - ↑ Barth H, Aktories K, Popoff MR, Stiles BG (2004). "Binary Bacterial Toxins: Biochemistry, Biology, and Applications of Common Clostridium and Bacillus Proteins". Microbiology and Molecular Biology Reviews : MMBR. 68 (3): 373–402, table of contents. doi:10.1128/MMBR.68.3.373-402.2004. PMC 515256
 . PMID 15353562.
. PMID 15353562. - ↑ Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M, Vibien A, Brassard P, Fenn S, Dewar K, Hudson TJ, Horn R, René P, Monczak Y, Dascal A (December 2005). "A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality". The New England Journal of Medicine. 353 (23): 2442–9. doi:10.1056/NEJMoa051639. PMID 16322602.
- ↑ McDonald LC (August 2005). "Clostridium difficile: responding to a new threat from an old enemy" (PDF). Infection Control and Hospital Epidemiology. 26 (8): 672–5. doi:10.1086/502600. PMID 16156321.
- ↑ Rupnik M, Wilcox MH, Gerding DN (July 2009). "Clostridium difficile infection: New developments in epidemiology and pathogenesis". Nature Reviews. Microbiology. 7 (7): 526–36. doi:10.1038/nrmicro2164. PMID 19528959.
- ↑ Luciano, JA; Zuckerbraun, BS (December 2014). "Clostridium difficile infection: prevention, treatment, and surgical management". The Surgical clinics of North America. 94 (6): 1335–49. doi:10.1016/j.suc.2014.08.006. PMID 25440127.
- ↑ "Scientists probe whether C. difficile is linked to eating meat". CBC News. 2006-10-04. Archived from the original on 24 October 2006.
- ↑ Clabots CR, Johnson S, Olson MM, Peterson LR, Gerding DN (September 1992). "Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection". The Journal of Infectious Diseases. 166 (3): 561–7. doi:10.1093/infdis/166.3.561. PMID 1323621.
- ↑ Halsey J (2008). "Current and future treatment modalities for Clostridium difficile-associated disease". American Journal of Health-System Pharmacy. 65 (8): 705–15. doi:10.2146/ajhp070077. PMID 18387898.
- ↑ Howell MD, Novack V, Grgurich P, Soulliard D, Novack L, Pencina M, Talmor D (May 2010). "Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection". Archives of Internal Medicine. 170 (9): 784–90. doi:10.1001/archinternmed.2010.89. PMID 20458086.
- ↑ Deshpande A, Pant C, Pasupuleti V, Rolston DD, Jain A, Deshpande N, Thota P, Sferra TJ, Hernandez AV (March 2012). "Association between proton pump inhibitor therapy and Clostridium difficile infection in a meta-analysis". Clinical Gastroenterology and Hepatology. 10 (3): 225–33. doi:10.1016/j.cgh.2011.09.030. PMID 22019794.
- ↑ Sarah A. Kuehne; Stephen T. Cartman; John T. Heap; Michelle L. Kelly; Alan Cockayne; Nigel P. Minton (2010). "The role of toxin A and toxin B in Clostridium difficile infection". Nature. 467 (7316): 711–3. doi:10.1038/nature09397. PMID 20844489.
- ↑ "Surgical Pathology Criteria: Pseudomembranous Colitis". Stanford School of Medicine.
- ↑ Murray PR, Baron EJ, Pfaller EA, Tenover F, Yolken RH, eds. (2003). Manual of Clinical Microbiology (8th ed.). Washington DC: ASM Press. ISBN 1-55581-255-4.
- ↑ Deshpande A, Pasupuleti V, Patel P, Ajani G, Hall G, Hu B, Jain A, Rolston DD (2011). "Repeat Stool Testing to Diagnose Clostridium difficile Infection Using Enzyme Immunoassay Does Not Increase Diagnostic Yield". Clinical Gastroenterology and Hepatology. 9 (8): 665–669.e1. doi:10.1016/j.cgh.2011.04.030. PMID 21635969.
- ↑ Anna Salleh (2009-03-02). "Researchers knock down gastro bug myths". ABC Science Online. Retrieved 2009-03-02.
- ↑ Lyras D, O'Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, Poon R, Adams V, Vedantam G, Johnson S, Gerding DN, Rood JI (2009). "Toxin B is essential for virulence of Clostridium difficile". Nature. 458 (7242): 1176–9. doi:10.1038/nature07822. PMC 2679968
 . PMID 19252482.
. PMID 19252482. - ↑ Deshpande A, Pasupuleti V, Rolston DD, Jain A, Deshpande N, Pant C, Hernandez AV (October 2011). "Diagnostic accuracy of real-time polymerase chain reaction in detection of Clostridium difficile in the stool samples of patients with suspected Clostridium difficile Infection: a meta-analysis.". Clinical Infectious Diseases. 53 (7): e81–90. doi:10.1093/cid/cir505. PMID 21890762.
- ↑ JOURNAL OF CLINICAL MICROBIOLOGY, Oct. 2010, p. 3738–3741
- ↑ Weiss AJ, Elixhauser A. Origin of Adverse Drug Events in U.S. Hospitals, 2011. HCUP Statistical Brief #158. Agency for Healthcare Research and Quality, Rockville, MD. July 2013.
- 1 2 Heineman J, Bubenik S, McClave S, Martindale R (August 2012). "Fighting fire with fire: is it time to use probiotics to manage pathogenic bacterial diseases?". Current Gastroenterology Reports. 14 (4): 343–8. doi:10.1007/s11894-012-0274-4. PMID 22763792.
- 1 2 Johnston BC, Ma SS, Goldenberg JZ, Thorlund K, Vandvik PO, Loeb M, Guyatt GH (18 December 2012). "Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis.". Annals of Internal Medicine. 157 (12): 878–88. doi:10.7326/0003-4819-157-12-201212180-00563. PMID 23362517.
- 1 2 3 4 5 Na X, Kelly C (November 2011). "Probiotics in Clostridium difficile Infection". Journal of Clinical Gastroenterology. 45 (Suppl): S154–8. doi:10.1097/MCG.0b013e31822ec787. PMID 21992956.
- ↑ McFarland LV (April 2006). "Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease". The American Journal of Gastroenterology. 101 (4): 812–22. doi:10.1111/j.1572-0241.2006.00465.x. PMID 16635227.
- ↑ Goldenberg JZ, Ma SS, Saxton JD, Martzen MR, Vandvik PO, Thorlund K, Guyatt GH, Johnston BC (31 May 2013). "Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children.". The Cochrane Database of Systematic Reviews. 5: CD006095. doi:10.1002/14651858.CD006095.pub3. PMID 23728658.
- ↑ Mayo Clinic C. diff prevention
- 1 2 Dubberke ER, Carling P, Carrico R, Donskey CJ, Loo VG, McDonald LC, Maragakis LL, Sandora TJ, Weber DJ, Yokoe DS, Gerding DN (Jun 2014). "Strategies to Prevent Clostridium difficile Infections in Acute Care Hospitals: 2014 Update.". Infection Control and Hospital Epidemiology. 35 (6): 628–45. doi:10.1086/676023. PMID 24799639.
- ↑ Roehr B (21 September 2007). "Alcohol Rub, Antiseptic Wipes Inferior at Removing Clostridium difficile". Medscape.
- ↑ Savidge TC, Urvil P, Oezguen N, Ali K, Choudhury A, Acharya V, Pinchuk I, Torres AG, English RD, Wiktorowicz JE, Loeffelholz M, Kumar R, Shi L, Nie W, Braun W, Herman B, Hausladen A, Feng H, Stamler JS, Pothoulakis C (2011). "Host S-nitrosylation inhibits clostridial small molecule–activated glucosylating toxins". Nature Medicine. 17 (9): 1136–41. doi:10.1038/nm.2405. PMC 3277400
 . PMID 21857653. Lay summary – ScienceDaily (21 August 2011).
. PMID 21857653. Lay summary – ScienceDaily (21 August 2011). - ↑ Laidman J (29 December 2011). "Flush With Germs: Lidless Toilets Spread C. difficile". Medscape.
- ↑ "Cleaning agents 'make bug strong'". BBC News Online. 2006-04-03. Retrieved 2008-11-17.
- ↑ Boyce et al. 2008
- ↑ Manian et al. 2010
- ↑ "Performance Feedback, Ultraviolet Cleaning Device, and Dedicated Housekeeping Team Significantly Improve Room Cleaning, Reduce Potential for Spread of Common, Dangerous Infection". Agency for Healthcare Research and Quality. 2014-01-15. Retrieved 2014-01-20.
- 1 2 Nelson RL, Kelsey P, Leeman H, Meardon N, Patel H, Paul K, Rees R, Taylor B, Wood E, Malakun R (7 September 2011). "Antibiotic treatment for Clostridium difficile-associated diarrhea in adults.". The Cochrane Database of Systematic Reviews (9): CD004610. doi:10.1002/14651858.CD004610.pub4. PMID 21901692.
- 1 2 Drekonja DM, Butler M, MacDonald R, Bliss D, Filice GA, Rector TS, Wilt TJ (20 December 2011). "Comparative effectiveness of Clostridium difficile treatments: a systematic review". Annals of Internal Medicine. 155 (12): 839–47. doi:10.7326/0003-4819-155-12-201112200-00007. PMID 22184691.
- 1 2 3 4 Surawicz, Christina M; Brandt, Lawrence J; Binion, David G; Ananthakrishnan, Ashwin N; Curry, Scott R; Gilligan, Peter H; McFarland, Lynne V; Mellow, Mark; et al. (26 February 2013). "Guidelines for Diagnosis, Treatment, and Prevention of Clostridium difficile Infections". The American Journal of Gastroenterology. 108 (4): 478–498. doi:10.1038/ajg.2013.4. PMID 23439232.
- ↑ Shen EP, Surawicz CM. Current Treatment Options for Severe Clostridium difficile–associated Disease. Gastroenterology & Hepatology. 2008;4(2):134-139.
- 1 2 Crawford T, Huesgen E, Danziger L (1 June 2012). "Fidaxomicin: a novel macrocyclic antibiotic for the treatment of Clostridium difficile infection.". American Journal of Health-System Pharmacy. 69 (11): 933–43. doi:10.2146/ajhp110371. PMID 22610025.
- ↑ Cornely OA (December 2012). "Current and emerging management options for Clostridium difficile infection: what is the role of fidaxomicin?". Clinical Microbiology and Infection. 18 Suppl 6: 28–35. doi:10.1111/1469-0691.12012. PMID 23121552.
- ↑ Cunha, Burke A. (2013). Antibiotic Essentials 2013 (12 ed.). p. 133. ISBN 978-1-284-03678-7.
- 1 2 Stroehlein JR (2004). "Treatment of Clostridium difficile Infection". Current Treatment Options in Gastroenterology. 7 (3): 235–9. doi:10.1007/s11938-004-0044-y. PMID 15149585.
- ↑ Bauer MP, van Dissel JT, Kuijper EJ (December 2009). "Clostridium difficile: controversies and approaches to management". Current Opinion in Infectious Diseases. 22 (6): 517–24. doi:10.1097/QCO.0b013e32833229ce. PMID 19738464.
- ↑ Pillai A, Nelson R (23 January 2008). Pillai, Anjana, ed. "Probiotics for treatment of Clostridium difficile-associated colitis in adults". The Cochrane Database of Systematic Reviews (1): CD004611. doi:10.1002/14651858.CD004611.pub2. PMID 18254055.
- ↑ Burke KE, Lamont JT (August 2013). "Fecal Transplantation for Recurrent Clostridium difficile Infection in Older Adults: A Review.". Journal of the American Geriatrics Society. 61 (8): 1394–8. doi:10.1111/jgs.12378. PMID 23869970.
- 1 2 Drekonja, D; Reich, J; Gezahegn, S; Greer, N; Shaukat, A; MacDonald, R; Rutks, I; Wilt, TJ (5 May 2015). "Fecal Microbiota Transplantation for Clostridium difficile Infection: A Systematic Review". Annals of Internal Medicine. 162 (9): 630–8. doi:10.7326/m14-2693. PMID 25938992.
- ↑ van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG, Keller JJ (January 2013). "Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile". The New England Journal of Medicine. 368 (5): 407–15. doi:10.1056/NEJMoa1205037. PMID 23323867.
- ↑ Jop De Vrieze (30 August 2011). "The Promise of Poop". Science. 341: 954–957. doi:10.1126/science.341.6149.954.
- ↑ Keller, JJ; Kuijper, EJ (2015). "Treatment of recurrent and severe Clostridium difficile infection". Annual Review of Medicine. 66: 373–86. doi:10.1146/annurev-med-070813-114317. PMID 25587656.
- ↑ Smith, Peter Andrey (10 November 2015). "Fecal Transplants Made (Somewhat) More Palatable". The New York Times. p. D5. Retrieved 13 November 2015.
- ↑ Bhangu A, Nepogodiev D, Gupta A, Torrance A, Singh P (November 2012). "Systematic review and meta-analysis of outcomes following emergency surgery for Clostridium difficile colitis". The British Journal of Surgery. 99 (11): 1501–13. doi:10.1002/bjs.8868. PMID 22972525.
- ↑ Osman KA, Ahmed MH, Hamad MA, Mathur D (October 2011). "Emergency colectomy for fulminant Clostridium difficile colitis: Striking the right balance". Scandinavian Journal of Gastroenterology. 46 (10): 1222–7. doi:10.3109/00365521.2011.605469. PMID 21843039.
- 1 2 Kelly CP, LaMont JT (October 2008). "Clostridium difficile—more difficult than ever". The New England Journal of Medicine. 359 (18): 1932–40. doi:10.1056/NEJMra0707500. PMID 18971494.
- 1 2 others], editor-in-chief, Frank J. Domino ; associate editors, Robert A. Baldor (2014). The 5-minute clinical consult 2014 (22nd ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 258. ISBN 978-1-4511-8850-9.
- ↑ "Antibiotic resistance threats in the United States, 2013" (PDF). US Centers for Disease Control and Prevention. 2013. Retrieved 3 November 2014.
- ↑ Hall IC, O'Toole E (1935). "Intestinal flora in newborn infants with a description of a new pathogenic anaerobe, Bacillus difficilis". American Journal of Diseases of Children. 49 (2): 390–402. doi:10.1001/archpedi.1935.01970020105010.
- ↑ Larson HE, Price AB, Honour P, Borriello SP (May 1978). "Clostridium difficile and the aetiology of pseudomembranous colitis". Lancet. 311 (8073): 1063–6. doi:10.1016/S0140-6736(78)90912-1. PMID 77366.
- ↑ "Trust confirms 49 superbug deaths". BBC News Online. 2006-10-01.
- ↑ Hawkes, Nigel (2007-01-11). "Leaked memo reveals that targets to beat MRSA will not be met" (snippet). The Times. London. Retrieved 2007-01-11.(subscription required)
- ↑ "C. difficile blamed for 9 death in hospital near Montreal". Canoe.ca. 27 October 2006. Retrieved 2007-01-11.
- ↑ "12th person dies of C. difficile at Quebec hospital". CBC News. 18 November 2006. Archived from the original on 21 October 2007.
- ↑ Hospitals struck by new killer bug An article by Manchester free newspaper 'Metro', 7 May 2008
- ↑ "C. difficile outbreak linked to fatal strain". CTV News. 28 February 2007.
- ↑ "Superbug in hospitals linked to four deaths". Irish Independent. 10 October 2007.
- ↑ "Welcome to the Public Inquiry into the Outbreak of Clostridium difficile in Northern Trust Hospitals"
- ↑ Healthcare watchdog finds significant failings in infection control at Maidstone and Tunbridge Wells NHS Trust (press release), United Kingdom: Healthcare Commission, 11 October 2007, archived from the original on 21 December 2007
- ↑ Smith, Rebecca; Rayner, Gordon; Adams, Stephen (11 October 2007). "Health Secretary intervenes in superbug row". Daily Telegraph. London.
- ↑ Ärhäkkä suolistobakteeri on tappanut jo kymmenen potilasta – HS.fi – Kotimaa
- ↑ "Possible C Diff link to Drogheda deaths". RTÉ News. 10 November 2009.
- ↑ 199 hit by the killer diarrhea at Herlev Hospital, BT 3 March 2010
- ↑ (Herlev, Amager, Gentofte and Hvidovre)
- ↑ Four hospitals affected by the dangerous bacterium, TV2 News 7 May 2010
- ↑ "Deadly superbug reaches NZ". 3 News NZ. 30 October 2012.
- ↑ "C. difficile linked to 26th death in Ontario". CBC News. 25 July 2011. Retrieved 24 July 2011.
- ↑ "10 punkter för att förhindra smittspridning i Region Skåne" [10 points to prevent the spread of infection in Region Skåne] (in Swedish). Archived from the original on 5 March 2015.
- ↑ Liddell-Scott. "κλωστήρ". Greek-English Lexicon. Oxford{{inconsistent citations}}
- ↑ Cawley, Kevin. "Difficilis". Latin Dictionary and Grammar Aid. University of Notre Dame. Retrieved 2013-03-16{{inconsistent citations}}
- ↑ Campus, University of Massachusetts Worcester. "op-line data from randomized, double-blind, placebo controlled Phase 2 clinical trial indicate statistically significant reduction in recurrences of CDAD". Archived from the original on 27 December 2010. Retrieved 2011-08-16
- ↑ CenterWatch. "Clostridium Difficile-Associated Diarrhea". Retrieved 2011-08-16.
- ↑ Business, Highbeam. "MDX 066, MDX 1388 Medarex, University of Massachusetts Medical School clinical data (phase II)(diarrhea)". Retrieved 2011-08-16.
- 1 2 3 4 5 Shah D, Dang MD, Hasbun R, Koo HL, Jiang ZD, DuPont HL, Garey KW (May 2010). "Clostridium difficile infection: update on emerging antibiotic treatment options and antibiotic resistance". Expert Review of Anti-Infective Therapy. 8 (5): 555–64. doi:10.1586/eri.10.28. PMC 3138198
 . PMID 20455684.
. PMID 20455684. - ↑ Moore, John H.; van Opstal, Edward; Kolling, Glynis L.; Shin, Jae Hyun; Bogatcheva, Elena; Nikonenko, Boris; Einck, Leo; Phipps, Andrew J.; Guerrant, Richard L. (2016-05-01). "Treatment of Clostridium difficile infection using SQ641, a capuramycin analogue, increases post-treatment survival and improves clinical measures of disease in a murine model". The Journal of Antimicrobial Chemotherapy. 71 (5): 1300–1306. doi:10.1093/jac/dkv479. ISSN 1460-2091. PMC 4830414
 . PMID 26832756.
. PMID 26832756. - ↑ Barlow, Andrew; Muhleman, Mitchel; Gielecki, Jerzy; Matusz, Petru; Tubbs, R. Shane; Loukas, Marios (2013). "The Vermiform Appendix: A Review". Clinical Anatomy. 26 (7): 833–842. doi:10.1002/ca.22269. PMID 23716128.
- ↑ Gerding, Dale N.; Meyer, Thomas; Lee, Christine; Cohen, Stuart H.; Murthy, Uma K.; Poirier, Andre; Van Schooneveld, Trevor C.; Pardi, Darrell S.; Ramos, Antonio; Barron, Michelle A.; Chen, Hongzi; Villano, Stephen (5 May 2015). "Administration of Spores of Nontoxigenic Strain M3 for Prevention of Recurrent Infection". JAMA. 313 (17): 1719. doi:10.1001/jama.2015.3725.
- ↑ , FDA Approves Merck’s ZINPLAVA™ (bezlotoxumab) to Reduce Recurrence of Clostridium difficile Infection (CDI) in Adult Patients Receiving Antibacterial Drug Treatment for CDI Who Are at High Risk of CDI Recurrence
External links
| Wikimedia Commons has media related to Clostridium difficile. |
- Pseudomembranous colitis at DMOZ
- Updated guidance on the management and treatment of Clostridium difficile infection