Carbon–carbon bond
A carbon–carbon bond is a covalent bond between two carbon atoms.[1] The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is formed between one hybridized orbital from each of the carbon atoms. In ethane, the orbitals are sp3-hybridized orbitals, but single bonds formed between carbon atoms with other hybridisations do occur (e.g. sp2 to sp2). In fact, the carbon atoms in the single bond need not be of the same hybridisation. Carbon atoms can also form double bonds in compounds called alkenes or triple bonds in compounds called alkynes. A double bond is formed with an sp2-hybridized orbital and a p-orbital that isn't involved in the hybridization. A triple bond is formed with an sp-hybridized orbital and two p-orbitals from each atom. The use of the p-orbitals forms a pi bond.
Carbon is one of the few elements that can form long chains of its own atoms, a property called catenation. This coupled with the strength of the carbon–carbon bond gives rise to an enormous number of molecular forms, many of which are important structural elements of life, so carbon compounds have their own field of study: organic chemistry.
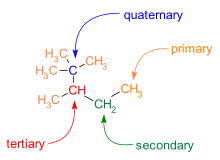
Branching is also common in C−C skeletons. Different carbon atoms can be identified with respect to the number of carbon neighbors:
- primary carbon atom: one carbon neighbor
- secondary carbon atom: two carbon neighbors
- tertiary carbon atom: three carbon neighbors
- quaternary carbon atom: four carbon neighbors
In "structurally complex organic molecules", it is the three-dimensional orientation of the carbon–carbon bonds at quaternary loci which dictates the shape of the molecule.[2] Further, quaternary loci are found in many biologically active small molecules, such as cortisone and morphine.[2]
Synthesis
Carbon–carbon bond-forming reactions are organic reactions in which a new carbon–carbon bond is formed. They are important in the production of many man-made chemicals such as pharmaceuticals and plastics.
Some examples of reactions which form carbon–carbon bonds are aldol reactions, Diels–Alder reaction, the addition of a Grignard reagent to a carbonyl group, a Heck reaction, a Michael reaction and a Wittig reaction.
The directed synthesis of desired three-dimensional structures for tertiary carbons was largely solved during the late 20th century, but the same ability to direct quaternary carbon synthesis did not start to emerge until the first decade of the 21st century.[2]
Bond strengths
Relative to most bonds, a carbon–carbon bond is very strong.[3]
| C–C bond | Molecule | Bond dissociation energy (kcal/mol) |
|---|---|---|
| CH3−CH3 | ethane | 90 |
| C5H5−CH3 | toluene | 102 |
| C5H5−C5H5 | biphenyl | 114 |
| CH3C(O)−CH3 | acetone | 84 |
| CH3−CN | acetonitrile | 136 |
| CH3−CH2OH | ethanol | 88 |
See also
- An extensive list is presented here: list of carbon–carbon bond-forming reactions
- The chemistry of carbon bonded to other elements in the periodic table:
| CH | He | ||||||||||||||||
| CLi | CBe | CB | CC | CN | CO | CF | Ne | ||||||||||
| CNa | CMg | CAl | CSi | CP | CS | CCl | CAr | ||||||||||
| CK | CCa | CSc | CTi | CV | CCr | CMn | CFe | CCo | CNi | CCu | CZn | CGa | CGe | CAs | CSe | CBr | CKr |
| CRb | CSr | CY | CZr | CNb | CMo | CTc | CRu | CRh | CPd | CAg | CCd | CIn | CSn | CSb | CTe | CI | CXe |
| CCs | CBa | CHf | CTa | CW | CRe | COs | CIr | CPt | CAu | CHg | CTl | CPb | CBi | CPo | CAt | Rn | |
| Fr | CRa | Rf | Db | CSg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| CLa | CCe | CPr | CNd | CPm | CSm | CEu | CGd | CTb | CDy | CHo | CEr | CTm | CYb | CLu | |||
| Ac | CTh | CPa | CU | CNp | CPu | CAm | CCm | CBk | CCf | CEs | Fm | Md | No | Lr | |||
| Core organic chemistry | Many uses in chemistry |
| Academic research, but no widespread use | Bond unknown |
References
- ↑ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- 1 2 3 Quasdorf, Kyle W.; Overman, Larry E. "Review: Catalytic enantioselective synthesis of quaternary carbon stereocentres". Nature (paper): 181–191. doi:10.1038/nature14007.

- ↑ Yu-Ran Luo and Jin-Pei Cheng "Bond Dissociation Energies" in CRC Handbook of Chemistry and Physics, 96th Edition.