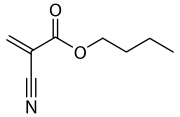
Butyl cyanoacrylate
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Butyl 2-cyanoprop-2-enoate | |
| Other names
Butyl 2-cyanopropenoate Butyl 2-cyanoacrylate 2-Cyano-2-propenoic acid n-butyl ester n-Butyl 2-cyanoacrylate n-BCA NBCA n-Butyl alpha-cyanoacrylate | |
| Identifiers | |
| 6606-65-1 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL2104251 |
| ChemSpider | 21607 |
| ECHA InfoCard | 100.026.866 |
| UNII | F8CEP82QNP |
| |
| |
| Properties | |
| C8H11NO2 | |
| Molar mass | 153.18 g/mol |
| Density | 1.4430 |
| Boiling point | 83 to 84 °C (181 to 183 °F; 356 to 357 K) at 3 mm Hg |
| Hazards | |
| Flash point | > 80 °C (176 °F; 353 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
n-Butyl cyanoacrylate (n-BCA, NBCA), a cyanoacrylate ester, is a butyl ester of 2-cyano-2-propenoic acid. It is a clear colorless liquid with a sharp, irritating odor. It is insoluble in water. Its chief use is as the main component of medical cyanoacrylate glues.[1] It can be encountered under various trade names, e.g. MediBond, MediCryl, PeriAcryl, GluStitch, Xoin, Gesika, VetGlu, Vetbond, LiquiVet, Indermil, LiquiBand, Histoacryl, IFABond and others.[2]
n-butyl-2-cyanoacrylate or butyl cyanoacrylate is also known as Embucrilate.
In medical and veterinary applications, n-butyl cyanoacrylate, isobutyl cyanoacrylate, and octyl cyanoacrylate are commonly used. They are bacteriostatic and their use is usually painless. Butyl esters provide stronger bond, but are rigid. Octyl esters, while providing weaker bond, are more flexible. Blends of octyl cyanoacrylate and n-butyl cyanoacrylate are available (such as GLUture) which offer both flexibility and a strong bond. n-Butyl cyanoacrylate is also used for embolization of cerebral arteriovenous malformations before their surgical treatment.[1]
It is soluble in acetone, methyl ethyl ketone, nitromethane, and methylene chloride.[3]
Butyl cyanoacrylate (in monomer form) polymerizes rapidly in presence of ionic substances like moisture, blood or tissue fluids.
Butyl cyanoacrylate has unique properties compared to other cyanoacrylates like octyl cyanoacrylate or iso amyl cyanoacrylate. Polymerized form has excellent tensile strength and is very effective in closing surgical or wound incisions.
The closure of the wound or cut is quick (about 30 to 45 seconds) and the product has inherently some valuable bacteriostatic properties. The cosmetic outcome of the closure is comparable or generally better than an equivalent suture substitute with least amount of scarring visible after three to six months.
Also important is the in the body degradation properties of polymerized butyl cyanoacrylate. This property of butyl cyanoacrylate has made it a very useful polymer to create various nano particles for delivery of drugs into the body with sustained release profiles.
Heating to higher temperatures causes pyrolysis and depolymerization of the cured glue, producing gaseous products strongly irritant to lungs and eyes.
Medical applications
The medical applications of butyl cyanoacrylate include its use as an adhesive for lacerations of the skin,[4] and in the treatment of bleeding from vascular structures. Butyl cyanoacrylate has been used to treat arteriovenous malformations[5] by application of the glue into the abnormality through angiography.
In gastroenterology, butyl cyanoacrylate is used to treat bleeding gastric varices, which are dilated veins that occur in the setting of liver cirrhosis or thrombosis of the splenic vein.[6] The gastric varices are accessed by endoscopy, which uses a flexible fibre-optic camera to enter the stomach. They are injected with a catheter needle inserted into the varix through the endoscope. Other sites of varices, including esophageal varices,[7] duodenal varices[8] and colonic varices.[9] Gastric varices have also been obliterated with recurrent injection treatment with butyl cyanoacrylate.[10]
See also
References
- 1 2 "n-Butyl-2-cyanoacrylate". Chemical Sampling Information. Washington, DC, USA: Occupational Safety & Health Administration. 17 January 2007. Retrieved 25 June 2011.
- ↑ "Material Safety Data Sheet for Butyl Octyl Blend" (PDF). GluStitch Inc. 19 October 2009. Archived from the original (PDF) on 26 March 2012. Retrieved 25 June 2011.
- ↑ http://www.palmlabsadhesives.com/technical_data.htm
- ↑ Farion K, Osmond MH, Hartling L, et al. (2002). Farion KJ, ed. "Tissue adhesives for traumatic lacerations in children and adults". Cochrane Database Syst Rev (3): CD003326. doi:10.1002/14651858.CD003326. PMID 12137689.
- ↑ Lee BB, Do YS, Yakes W, Kim DI, Mattassi R, Hyon WS (March 2004). "Management of arteriovenous malformations: a multidisciplinary approach". J. Vasc. Surg. 39 (3): 590–600. doi:10.1016/j.jvs.2003.10.048. PMID 14981454.
- ↑ Ferguson JW, Tripathi D, Hayes PC (August 2003). "Review article: the management of acute variceal bleeding". Aliment. Pharmacol. Ther. 18 (3): 253–62. doi:10.1046/j.1365-2036.2003.01664.x. PMID 12895210.
- ↑ D'Imperio N, Piemontese A, Baroncini D, et al. (February 1996). "Evaluation of undiluted N-butyl-2-cyanoacrylate in the endoscopic treatment of upper gastrointestinal tract varices". Endoscopy. 28 (2): 239–43. doi:10.1055/s-2007-1005435. PMID 8739740.
- ↑ Ota K, Shirai Z, Masuzaki T, et al. (August 1998). "Endoscopic injection sclerotherapy with n-butyl-2-cyanoacrylate for ruptured duodenal varices". J. Gastroenterol. 33 (4): 550–5. doi:10.1007/s005350050131. PMID 9719241.
- ↑ Chen WC, Hou MC, Lin HC, Chang FY, Lee SD (February 2000). "An endoscopic injection with N-butyl-2-cyanoacrylate used for colonic variceal bleeding: a case report and review of the literature". Am. J. Gastroenterol. 95 (2): 540–2. doi:10.1111/j.1572-0241.2000.01782.x. PMID 10685765.
- ↑ Lo GH, Lai KH, Cheng JS, Chen MH, Chiang HT (May 2001). "A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices". Hepatology. 33 (5): 1060–4. doi:10.1053/jhep.2001.24116. PMID 11343232.