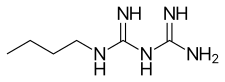
Buformin
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | A10BA03 (WHO) |
| Pharmacokinetic data | |
| Excretion | Renal |
| Identifiers | |
| CAS Number |
692-13-7 |
| PubChem (CID) | 2468 |
| DrugBank |
DB04830 |
| ChemSpider |
2374 |
| UNII |
W2115E9C7B |
| KEGG |
D00595 |
| ChEMBL |
CHEMBL39736 |
| ECHA InfoCard | 100.010.662 |
| Chemical and physical data | |
| Formula | C6H15N5 |
| Molar mass | 157.22 g·mol−1 |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Buformin (1-butylbiguanide) is an oral antidiabetic drug of the biguanide class, chemically related to metformin and phenformin. Buformin was marketed by German pharmaceutical company Grünenthal as Silubin.
Chemistry and animal toxicology
Buformin hydrochloride is a fine, white to slightly yellow, crystalline, odorless powder, with a weakly acidic bitter taste. Its melting point is 174 to 177 °C, it is a strong base, and is freely soluble in water, methanol and ethanol, but insoluble in chloroform and ether.[1][2] Toxicity: guinea pig LD50 subcutaneous 18 mg/kg; mouse LD50 intraperitoneal 140 mg/kg and 300 mg/kg oral.[3] The partition coefficient (log P in octanol-water) is -1.20E+00; its water solubility is 7.46E+05 mg/l at 25 °C. Vapor pressure is 1.64E-04 mm Hg at 25 °C (EST); Henry's law constant is 8.14E-16 atm-m3/mole at 25 °C (EST). Its Atmospheric -OH rate constant is 1.60E-10 cm3/molecule-sec at 25 °C.[4]
Mechanism of action
Buformin delays absorption of glucose from the gastrointestinal tract, increases insulin sensitivity and glucose uptake into cells, and inhibits synthesis of glucose by the liver. Buformin and the other biguanides are not hypoglycemic, but rather antihyperglycemic agents. They do not produce hypoglycemia; instead, they reduce basal and postprandial hyperglycemia in diabetics.[5] Biguanides may antagonize the action of glucagon, thus reducing fasting glucose levels.[6]
Pharmacokinetics
After oral administration of 50 mg of buformin to volunteers, almost 90% of the applied quantity was recovered in the urine; the rate constant of elimination was found to be 0.38 per hr. Buformin is a strong base (pKa = 11.3) and not absorbed in the stomach. After intravenous injection of about 1 mg/kg buformin-14-C, the initial serum concentration is 0.2-0.4 µg/ml. Serum level and urinary elimination rate are linearly correlated.[7] In man, after oral administration of 50 mg 14-C-buformin, the maximum serum concentration was 0.26-0.41 µg/ml. The buformin was eliminated with an average half-life of 2 h. About 84% of the dose administered was found excreted unchanged in the urine.[8] Buformin is not metabolized in humans. The bioavailability of oral buformin and other biguanides is 40%-60%. Binding to plasma proteins is absent or very low.[9][10][11]
Dosage
The daily dose of buformin is 150–300 mg by mouth.[12] Buformin has also been available in a sustained release preparation, Silubin Retard, which is still sold in Romania.
Side effects and contraindications
The side effects encountered are anorexia, nausea, diarrhea, metallic taste, and weight loss. Its use is contraindicated in diabetic coma, ketoacidosis, severe infection, trauma, other conditions where buformin is unlikely to control the hyperglycemia, renal or hepatic impairment, heart failure, recent myocardial infarct, dehydration, alcoholism, and conditions likely to predispose to lactic acidosis.
Toxicity
Buformin was withdrawn from the market in many countries due to an elevated risk of causing lactic acidosis (although not the US, where it was never sold). Buformin is still available and prescribed in Romania (timed release Silubin Retard is sold by Zentiva), Hungary,[13][14][15][16] Taiwan[17] and Japan.[18] The lactic acidosis occurred only in patients with a buformin plasma level of greater than 0.60 µg/ml and was rare in patients with normal renal function.[19][20][21] In one report, the toxic oral dose was 329 ± 30 mg/day in 24 patients who developed lactic acidosis on buformin. Another group of 24 patients on 258 ± 25 mg/day did not develop lactic acidosis on buformin.[22]
Anticancer properties
Buformin, along with phenformin and metformin, inhibits the growth and development of cancer.[23][24][25][26][27] The anticancer property of these drugs is due to their ability to disrupt the Warburg effect and revert the cytosolic glycolysis characteristic of cancer cells to normal oxidation of pyruvate by the mitochondria.[28] Metformin reduces liver glucose production in diabetics and disrupts the Warburg effect in cancer by AMPK activation and inhibition of the mTor pathway.[29] Buformin decreased cancer incidence, multiplicity, and burden in chemically induced rat mammary cancer, whereas metformin and phenformin had no statistically significant effect on the carcinogenic process relative to the control group.[30] Buformin also exhibits anti-proliferative and anti-invasive effects in endometrial cancer cells.[31]
History
Buformin was synthesized as an oral antidiabetic in 1957.[32]
Synthesis
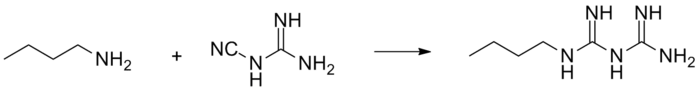
Buformin is obtained by reaction of butylamine and 2-cyanoguanidine.
References
- ↑ Jacker, HJ (1964). "New Pharmacologic Products. 2. Buformin For Oral Therapy Of Diabetes". Pharm Prax. 10: 247–9.
- ↑ Eustace George Coverly Clarke, Judith Berle, Pharmaceutical Society of Great Britain. Dept. of Pharmaceutical Sciences. Isolation and identification of drugs in pharmaceuticals, body fluids and post-mortem material, Volume 1. Pharmaceutical Press 1974, p226
- ↑ Shroff, JR; Bandurco, V; Desai, R; Kobrin, S; Cervoni, P (Dec 1981). "Chemistry and hypoglycemic activity of benzimidoylpyrazoles". J Med Chem. 24 (12): 1521–5. doi:10.1021/jm00144a031.
- ↑ United States National Library of Medicine ChemLDplus advanced database
- ↑ Enrique Ravina, Hugo Kubinyi. The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. Wiley. 2011 p 215
- ↑ Miller, RA; Chu, Q; Xie, J; Foretz, M; Viollet, B; Birnbaum, MJ (Feb 2013). "Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP". Nature. 494 (7436): 256–60. doi:10.1038/nature11808. PMID 23292513.
- ↑ Beckmann, R (Mar 1968). "The fate of biguanides in man". Ann N Y Acad Sci. 148 (3): 820–32.
- ↑ Beckmann, R; Lintz, W; Schmidt-Böthelt, E (Sep 1971). "Evaluation of a sustained release form of the oral antidiabetic butylbiguanide (Silubin retard)". Eur J Clin Pharmacol. 3 (4): 221–8. doi:10.1007/bf00565010.
- ↑ Marchetti, P; Giannarelli, R; Carlo, A; Navalesi, R (Oct 1991). "Pharmacokinetic optimisation of oral hypoglycaemic therapy". Clin Pharmacokinet. 21 (4): 308–17. doi:10.2165/00003088-199121040-00006. PMID 1760902.
- ↑ Gutsche, H; Blumenbach, L; Losert, W; Wiemann, H (1976). "Concentration of 14C-1-butylbiguanide in plasma of diabetic patients and its elimination after administration of a new Galenical formulation". Arzneimittelforschung. 26 (6): 1227–9.
- ↑ Ritzl, F; Feinendegen, LE; Lintz, W; Tisljar, U (1978). "Distribution and excretion of 14c-butylbiguanide in man". Arzneimittelforschung. 28 (7): 1184–6.
- ↑ Gustav Kuschinsky, Heinz Lüllmann. Textbook of pharmacology. Academic Press p 225, 1973
- ↑ Hankó, B; Tukarcs, E; Kumli, P; Vincze, Z (Jun 2005). "Antidiabetic drug utilization in Hungary". Pharm World Sci. 27 (3): 263–5. doi:10.1007/s11096-004-5804-1.
- ↑ Hankó BZ, Reszegi CA, Kumli P, Vincze Z. [Practice of antidiabetic therapy in Hungary]. Acta Pharm Hung. 2005;75(2):77-86.
- ↑ Jerry L. Schlesser, Gale Research Inc. Drugs available abroad. Derwent Publications, Ltd - 1990 p28
- ↑ Verdonck L, Sangster B, van Heijst A, de Groot G, Maes R (1981). "Buformin concentrations in a case of fatal lactic acidosis". Diabetologia. 20 (1): 45–6. doi:10.1007/BF01789112. PMID 7202882.
- ↑ Chou, CH; Cheng, CL; Huang, CC (May 2004). "A validated HPLC method with ultraviolet detection for the determination of buformin in plasma". Biomed Chromatogr. 18 (4): 254–8. doi:10.1002/bmc.312.
- ↑ Takeda Announces Submission Of Application For Additional Indication Of Actos In Japan; Concomitant Therapy With Biguanides For Type 2 Diabetes. Medical News Today. 28 Jan 2007
- ↑ Wittmann P, Haslbeck M, Bachmann W, Mehnert H. [Lactic acidosis in diabetics on biguanides (author's translation)] Deutsche Medizinische Wochenschrift 102(1):5-10, 1977
- ↑ Berger, W; Mehnert-Aner, S; Mülly, K; Heierli, C; Ritz, R (1976). "[10 cases of lactic acidosis during biguanide therapy (buformin and phenformin)].". Schweizerische medizinische Wochenschrift. 106: 1830–1834.
- ↑ Deppermann D, Heidland A, Ritz E, Hörl W (1978). "[Lactic acidosis--a possible complication in buformin-treated diabetics (author's transl)]". Klin Wochenschr. 56 (17): 843–53. PMID 713413.
- ↑ Luft, D; Schmülling, RM; Eggstein, M (Feb 1978). "Lactic acidosis in biguanide-treated diabetics: a review of 330 cases". Diabetologia. 14 (2): 75–87. doi:10.1007/bf01263444.
- ↑ Saito, Sakae; Furuno, Aki; Sakurai, Junko; Sakamoto, Asami; Park, Hae-Ryong; Shin-ya, Kazuo; Tsuruo, Takashi; Tomida, Akihiro (2009). "Chemical Genomics Identifies the Unfolded Protein Response as a Target for Selective Cancer Cell Killing during Glucose Deprivation". Cancer Research. 69 (10): 4225–34. doi:10.1158/0008-5472.can-08-2689.
- ↑ Vladimir N. Anisimov. "Insulin/IGF-1 signaling pathway driving aging and cancer as a target for pharmacological intervention. Experimental Gerontology Volume 38, Issue 10, October 2003, Pages 1041-1049
- ↑ Alexandrov, Valery A.; Anisimov, Vladimir N.; Belous, Natalia M.; Vasilyeva, Inna A.; Mazon, Vera B. (1980). "The inhibition of the transplacental blastomogenic effect of nitrosomethylurea by postnatal administration of buformin to rats". Carcinogenesis. 1 (12): 975–978. doi:10.1093/carcin/1.12.975.
- ↑ Anisimov, VN; Ostroumova, MN; Dil'man, VM (1980). "Inhibition of the blastomogenic effect of 7,12-dimethylbenz(a)anthracene in female rats by buformin, diphenin, a polypeptide pineal extract and L-DOPA". Bulletin of Experimental Biology and Medicine. 89 (6): 819–822. doi:10.1007/bf00836263.
- ↑ Anisimov, Vladimir N.; Berstein, Lev M.; Popovich, Irina G.; Zabezhinski, Mark A.; Egormin, Peter A.; Tyndyk, Margarita L.; Anikin, Ivan V.; Semenchenko, Anna V.; Yashin, Anatoli I. (2005). "Central and Peripheral Effects of Insulin/IGF-1 Signaling in Aging and Cancer: Antidiabetic Drugs as Geroprotectors and Anticarcinogens". Annals of the New York Academy of Sciences. 1057: 220–234. doi:10.1196/annals.1356.017.
- ↑ Vander Heiden, Matthew G.; Cantley, Lewis C.; Thompson, Craig B. (2009). "Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation". Science. 324 (5930): 1029–1033. doi:10.1126/science.1160809. PMC 2849637
 . PMID 19460998.
. PMID 19460998. - ↑ Shaw, RJ; Lamia, KA; Vasquez, D; Koo, SH; Bardeesy, N; Depinho, RA; Montminy, M; Cantley, LC (Dec 2005). "The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin". Science. 310 (5754): 1642–6. doi:10.1126/science.1120781.
- ↑ Zhu Z, Jiang W, Thompson MD, Echeverria D, McGinley JN, Thompson HJ. Effects of metformin, buformin, and phenformin on the post-initiation stage of chemically induced mammary carcinogenesis in the rat. Cancer Prev Res (Phila). 2015 Jun;8(6):518-27. doi: 10.1158/1940-6207.CAPR-14-0121. Epub 2015 Mar 24. PMID 25804611
- ↑ Kilgore J, Jackson AL, Clark LH, Guo H, Zhang L, Jones HM, Gilliam TP, Gehrig PA, Zhou C, Bae-Jump VL. Buformin exhibits anti-proliferative and anti-invasive effects in endometrial cancer cells. Am J Transl Res. 2016 Jun 15;8(6):2705-15. eCollection 2016. PMID 27398153
- ↑ Seymour L. Shapiro et al. Salts Of N-Amylbiguanide. US Patent number: 2961377; Filing date: Aug 5, 1957; Issue date: 1960
- ↑ Shapiro, S. L.; Parrino, V. A.; Freedman, L. (1959). "Hypoglycemic Agents. III.1—3N1-Alkyl- and Aralkylbiguanides". Journal of the American Chemical Society. 81 (14): 3728–3736. doi:10.1021/ja01523a060.