Halohydrin

In organic chemistry a halohydrin (also a haloalcohol or β-halo alcohol) is a functional group in which a halogen and a hydroxyl are bonded to adjacent carbon atoms, which otherwise bear only hydrogen or hydrocarbyl groups (e.g. 2-chloroethanol, 3-chloropropane-1,2-diol).[1] The term only applies to saturated motifs, as such compounds like 2-chlorophenol would not normally be considered halohydrins. Megatons of some chlorohydrins, e.g. propylene chlorohydrin, are produced annually as precursors to polymers.
Halohydrins may be categorized as chlorohydrins, bromohydrins, fluorohydrins or iodohydrins depending on the halogen present.
Synthesis
Halohydrins are usually prepared by treatment of an alkene with a halogen, in the presence of water.[2] The reaction is a form of electrophilic addition, similar to the halogen addition reaction and proceeds with anti addition, leaving the newly added X and OH groups in a trans configuration. The chemical equation for the conversion of ethylene to ethylene chlorohydrin is:
- H2C=CH2 + X2 + H2O → H2C-CH2Cl
When bromination is desired, N-bromosuccinimide (NBS) can be preferable to bromine because fewer side-products are produced.

Halohydrins may also be prepared from the reaction of an epoxide with a hydrohalic acid, or a metal halide.[3]
The reaction is produced on a an industrial scale for the production of chlorohydrin precursors to two important epoxides, epichlorohydrin and propylene oxide.[4]
Reactions
In presence of a base a halohydrin undergo internal SN2 reaction to form an epoxides. Industrially, the base is calcium hydroxide, whereas in the laboratory, potassium hydroxide is often used.
This reaction is the reverse of the formation reaction from an epoxide and can be considered a variant of the Williamson ether synthesis. Most of the world's supply of propylene oxide arises via this route.[5]
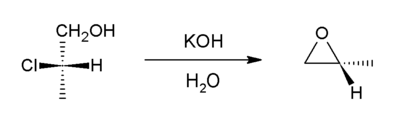
Such reactions can form the basis of more complicated processes, for example epoxide formation is one of the key steps in the Darzens reaction.
Halogenated halohydrins

Compounds such as 2,2,2-trichloroethanol, which contain several geminal halogens adjacent to a hydroxyl group may be considered halohydrins (although, strictly speaking, they fail the IUPAC definition) as they possess similar chemistry. In particular they also undergo intramolecular cyclisation to form dihaloepoxy groups. These species are both highly reactive and synthetically useful, forming the basis of the Jocic-Reeve, Bargellini and Corey–Link reactions.[6]
Safety
As with any functional group, the hazards of halohydrins are difficult to generalize as they may form part of an almost limitless series of compounds, with each structure having different pharmacology. In general, simpler low molecular weight compounds are often toxic and carcinogenic (e.g. 2-chloroethanol, 3-MCPD) by virtue of being alkylating agents. This reactivity can be put to good use, for instance in the anti-cancer drug mitobronitol. A number of synthetic corticosteroids exist baring a fluorohydrin motif (triamcinolone, dexamethasone).
Misnomers
Despite their rather suggestive names epichlorohydrin and sulfuric chlorohydrin are not halohydrins.
See also
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "halohydrins".
- ↑ William Reusch. "Addition Reactions of Alkenes". Virtual Textbook of Organic Chemistry. External link in
|work=(help) - ↑ Bonini, Carlo; Righi, Giuliana (1994). "Regio- and Chemoselective Synthesis of Halohydrins by Cleavage of Oxiranes with Metal Halides". Synthesis. 1994 (03): 225–238. doi:10.1055/s-1994-25445.
- ↑ Gordon Y. T. Liu, W. Frank Richey, Joanne E. Betso, Brian Hughes, Joanna Klapacz and Joerg Lindner "Chlorohydrins" in Ullmann's Encyclopedia of Industrial Chemistry, 2014 by Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_565.pub2
- ↑ Dietmar Kahlich, Uwe Wiechern, Jörg Lindner “Propylene Oxide” in Ullmann's Encyclopedia of Industrial Chemistry, 2002 by Wiley-VCH, Weinheim. doi:10.1002/14356007.a22_239 Article Online Posting Date: June 15, 2000
- ↑ Snowden, T.S. (28 February 2012). "Recent applications of gem-dichloroepoxide intermediates in synthesis". Arkivoc. 2012 (2): 24–40. doi:10.3998/ark.5550190.0013.204.