Binimetinib
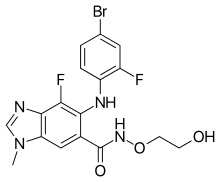 | |
| Clinical data | |
|---|---|
| ATC code | None |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | 606143-89-9 |
| PubChem (CID) | 10288191 |
| ChemSpider | 8463660 |
| KEGG | D10604 |
| ChEMBL | CHEMBL3187723 |
| Chemical and physical data | |
| Formula | C17H15BrF2N4O3 |
| Molar mass | 441.23 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Binimetinib (MEK162, ARRY-162) is a MEK inhibitor being developed by Array Biopharma to treat various cancers.[1]
It can be taken orally in tablet form.[2]
In 2015 it was in phase 3 clinical trials for ovarian cancer,[3] BRAF mutant melanoma,[4] and NRAS Q61 mutant melanoma.[2]
In Dec 2015 the company announced that the mutant-NRAS melanoma trial was successful.[5] In the trial, those receiving binimetinib had a median progression-free survival of 2.8 months versus 1.5 months for those on the standard dacarbazine treatment.[6] NDA submitted Jun 2016,[7] and the FDA should decide by 30 June 2017.[8]
In April 2016 it was reported that the phase III trial for low-grade ovarian cancer was terminated due to lack of efficacy.[9]
References
- ↑ "Array biopharma:: Binimetinib"
- 1 2 Study Comparing the Efficacy of MEK162 Versus Dacarbazine in Unresectable or Metastatic NRAS Mutation-positive Melanoma
- ↑ A Study of MEK162 vs. Physician's Choice Chemotherapy in Patients With Low-grade Serous Ovarian, Fallopian Tube or Peritoneal Cancer
- ↑ Study Comparing Combination of LGX818 Plus MEK162 Versus Vemurafenib and LGX818 Monotherapy in BRAF Mutant Melanoma (COLUMBUS)
- ↑ Array BioPharma Has Successful Trial for Cancer Drug Binimetinib. Dec 2015
- ↑ Array BioPharma announces Phase 3 binimetinib trial meets primary endpoint for NRAS-mutant melanoma. Dec 2015
- ↑ Array Bio submits marketing application in U.S. for lead product candidate in certain type of melanoma. June 2016
- ↑ FDA accepts Array Bio's NDA for binimetinib, action date June 30; Sept 2016
- ↑ Array bags Phase 3 study of binimetinib in ovarian cancer; shares down 4%
This article is issued from Wikipedia - version of the 11/1/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.