Barium cyanide
 | |
| Names | |
|---|---|
| IUPAC name
Barium dicyanide | |
| Identifiers | |
| 542-62-1 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 10496 |
| ECHA InfoCard | 100.008.021 |
| EC Number | 208-882-3 |
| PubChem | 10961 |
| |
| |
| Properties | |
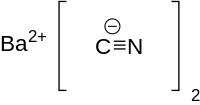
| Ba(CN)2 | |
| Molar mass | 189.362 g/mol |
| Appearance | white crystal |
| Melting point | 600 °C (1,112 °F; 873 K) |
| 18 g/100 mL (14 °C) | |
| Solubility | Soluble in ethanol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Barium cyanide is a chemical compound with the formula Ba(CN)2. It is synthesized by the reaction of hydrogen cyanide and barium hydroxide in water or petroleum ether.[1] This white crystal reacts with water and carbon dioxide in air slowly, producing highly toxic hydrogen cyanide gas.[2]
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
- ↑ Smith, R P; Gosselin, R E (1976). "Current Concepts about the Treatment of Selected Poisonings: Nitrite, Cyanide, Sulfide, Barium, and Quinidine". Annual Review of Pharmacology and Toxicology. 16: 189–99. doi:10.1146/annurev.pa.16.040176.001201. PMID 779614.
| Salts and covalent derivatives of the cyanide ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCN | He | ||||||||||||||||||
| LiCN | Be(CN)2 | B | C | NH4CN | OCN−, -NCO |
FCN | Ne | ||||||||||||
| NaCN | Mg(CN)2 | Al(CN)3 | SiCN | P(CN)3 | SCN−, -NCS, (SCN)2, S(CN)2 |
ClCN | Ar | ||||||||||||
| KCN | Ca(CN)2 | Sc(CN)3 | Ti(CN)4 | VO(CN)3 | Cr(CN)3 | Mn(CN)2 | Fe(CN)3, Fe(CN)64+, Fe(CN)63+ |
Co(CN)2, Co(CN)3 |
Ni(CN)2 Ni(CN)42− |
CuCN | Zn(CN)2 | Ga(CN)3 | Ge | As(CN)3 | SeCN− (SeCN)2 Se(CN)2 |
BrCN | Kr | ||
| RbCN | Sr(CN)2 | Y(CN)3 | Zr(CN)4 | Nb | Mo | Tc | Ru | Rh | Pd(CN)2 | AgCN | Cd(CN)2 | In(CN)3 | Sn | Sb | Te(CN)2, Te(CN)4 |
ICN | XeCN | ||
| CsCN | Ba(CN)2 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2(CN)2, Hg(CN)2 |
TlCN | Pb(CN)2 | Bi(CN)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La | Ce(CN)3, Ce(CN)4 |
Pr | Nd | Pm | Sm | Eu | Gd(CN)3 | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||||
| Ac | Th | Pa | UO2(CN)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
This article is issued from Wikipedia - version of the 6/17/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.