Antillatoxin
 | |
 Three-dimensional representation of antillatoxin | |
| Names | |
|---|---|
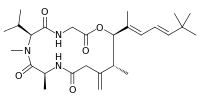
| IUPAC name
(6S,9S,14R,15R)-7,9,14-Trimethyl-13-methylidene-6-propan-2-yl-15-[(2E,4E)-4,6,6-trimethylhepta-2,4-dien-2-yl]-1-oxa-4,7,10-triazacyclopentadecane-2,5,8,11-tetrone | |
| Other names
ATX | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 28287038 |
| PubChem | 10051827 |
| |
| |
| Properties | |
| C28H45N3O5 | |
| Molar mass | 503.674 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Antillatoxin (ATX) is a potent lipopeptide neurotoxin produced by the marine cyanobacterium Lyngbya majuscula. ATX activates voltage-gated sodium channels, which can cause cell depolarisation, NMDA-receptor overactivity, excess calcium influx and neuronal necrosis.
Sources
Antillatoxin is found in the venom of the marine cyanobacterium Lyngbya majuscula. This cyanobacterium has a worldwide distribution throughout the tropics and subtropics in water up to 30 m depth.[1]
Structure
The three dimensional NMR study of this toxin showed that it consists of a tripeptide glycine-N-methylvaline-alanine, a hydroxycarboxylic acid and a 9-t-butyl-6,8-dimethyl-6,8-diene attached to the C5 atom of the cyclic peptide backbone.[2][3]
Analogs
There are three known analogous structures of ATX which have different toxicity: antillatoxin B (8-demethyl-antillatoxin) and DH-ATX (8-demethyl-8,9-dihydro-antillatoxin).[4][5]
Target
Antillatoxin is a sodium channel gating modifier with special efficacy in cells expressing rNav1.2, rNav1.4 and rNav1.5 α subunits.[5] It is suggested that ATX preferentially binds to the voltage-gated sodium channel in the inactivated state.[5] The specific site of interaction of this neurotoxin is not yet known, however there is an allosteric interaction between ATX and brevetoxin (PbTx) at site 5 of the α subunit, which indicates that the neurotoxin site for ATX is topologically close and/or conformationally coupled to neurotoxin site 5.[6] Additionally, sites 1, 2, 3, 5 and 7 were ruled out as possible binding sites.
Changing the tert-butyl-substituted diene groups reduced toxicity, which proves that the twisted shape of these groups plays a critical role in the degree of neurotoxicity of ATX.[7]
Mode of action
Antillatoxin activates voltage-gated sodium channels, thus increasing sodium influx into the cell.[6][8] It is hypothesized that ATX creates the increase in sodium influx by altering the voltage-gating properties of the channel. The toxin might change the voltage dependence of inactivation or augment the rate of recovery from inactivation.[5] The effect is concentration dependent, with similar potency for the rNav1.2,rNav1.4 and rNav1.5 α-subunit types of sodium channels.[5]
Antillatoxin-induced cytotoxicity is thought to occur through excessive activation of NMDA receptors by increased sodium influx, leading to excess calcium influx and necrosis.[6] This seems plausible as cytotoxicity can be prevented by either tetrodotoxin[6] or NMDA-receptor antagonists if administered early after exposure.[9] Yet the exact mechanism is still unclear, as antillatoxin’s effect on the membrane potential is not sufficient to relieve the NMDA receptor block by magnesium.[8]
Aside from toxic effects, ATX seems to enhance neurite outgrowth in developing immature neurons, depending on sodium influx, NMDA receptor activity, voltage-gated calcium channels and the calmodulin-kinase pathway.[8]
Antillatoxin increases the binding affinity of voltage-gated sodium channels for Batrachotoxin at site 5 in an allosteric way, possibly by inducing a conformational change favourable for Batrachotoxin binding.[6]
Toxicity
The toxin has been implicated in cases of respiratory irritation, inflammation of the eye and severe contact dermatitis in fishermen.[10] Antillatoxin is a very potent neurotoxin,[2] although exact toxicity differs between species. The lethal concentration LC50 is about 0.1 µM for goldfish,[4] making it the most potent toxin known for goldfish after brevetoxin.[2] It can be cytotoxic to single cerebellar granule cells at concentrations as low as 20 nM in rats[9] but more typically at 50 nM.[3]
Morphological features of antillatoxin-induced neuronal toxicity are swelling of neuronal somata, thinning of neurites and blebbing of neurite membranes.[9]
References
- ↑ Osborne, Nicholas J. T.; Webb, Penny M.; Shaw, Glen R. (2001-11-01). "The toxins of Lyngbya majuscula and their human and ecological health effects". Environment International. 27 (5): 381–392. doi:10.1016/S0160-4120(01)00098-8.
- 1 2 3 Orjala, Jimmy; Nagle, Dale G.; Hsu, Victor; Gerwick, William H. (1995-08-01). "Antillatoxin: An Exceptionally Ichthyotoxic Cyclic Lipopeptide from the Tropical Cyanobacterium Lyngbya majuscula". Journal of the American Chemical Society. 117 (31): 8281–8282. doi:10.1021/ja00136a031. ISSN 0002-7863.
- 1 2 Inoue, Masayuki (2014-01-01). "Chemical construction and structural permutation of neurotoxic natural product, antillatoxin: importance of the three-dimensional structure of the bulky side chain". Proceedings of the Japan Academy, Series B. 90 (2): 56–66. doi:10.2183/pjab.90.56. PMC 3948940
 . PMID 24522155.
. PMID 24522155. - 1 2 Nogle, Lisa M.; Okino, Tatsufumi; Gerwick, William H. (2001-07-01). "Antillatoxin B, a Neurotoxic Lipopeptide from the Marine Cyanobacterium Lyngbya majuscula". Journal of Natural Products. 64 (7): 983–985. doi:10.1021/np010107f. ISSN 0163-3864.
- 1 2 3 4 5 Cao, Zhengyu; Gerwick, William H.; Murray, Thomas F. (2010-12-14). "Antillatoxin is a sodium channel activator that displays unique efficacy in heterologously expressed rNav1.2, rNav1.4 and rNav1.5 alpha subunits". BMC Neuroscience. 11 (1): 154. doi:10.1186/1471-2202-11-154. ISSN 1471-2202. PMC 3009643
 . PMID 21156065.
. PMID 21156065. - 1 2 3 4 5 Li, W. I.; Berman, F. W.; Okino, T.; Yokokawa, F.; Shioiri, T.; Gerwick, W. H.; Murray, T. F. (2001-06-19). "Antillatoxin is a marine cyanobacterial toxin that potently activates voltage-gated sodium channels". Proceedings of the National Academy of Sciences. 98 (13): 7599–7604. doi:10.1073/pnas.121085898. ISSN 0027-8424. PMC 34714
 . PMID 11416227.
. PMID 11416227. - ↑ Okura, Ken; Matsuoka, Shigeru; Goto, Ryosuke; Inoue, Masayuki (2010). "The Twisted Side Chain of Antillatoxin is Important for Potent Toxicity: Total Synthesis and Biological Evaluation of Antillatoxin and Analogues.". Angewandte Chemie International Edition in English. 49 (2): 329–332. doi:10.1002/anie.200905892. PMID 19998300.
- 1 2 3 Jabba, S. V.; Prakash, A.; Dravid, S. M.; Gerwick, W. H.; Murray, T. F. (2010-03-01). "Antillatoxin, a Novel Lipopeptide, Enhances Neurite Outgrowth in Immature Cerebrocortical Neurons through Activation of Voltage-Gated Sodium Channels". Journal of Pharmacology and Experimental Therapeutics. 332 (3): 698–709. doi:10.1124/jpet.109.161802. ISSN 1521-0103. PMC 2835437
 . PMID 20026674.
. PMID 20026674. - 1 2 3 Berman, F. W; Gerwick, W. H; Murray, T. F (1999-11-01). "Antillatoxin and kalkitoxin, ichthyotoxins from the tropical cyanobacterium Lyngbya majuscula, induce distinct temporal patterns of NMDA receptor-mediated neurotoxicity". Toxicon. 37 (11): 1645–1648. doi:10.1016/S0041-0101(99)00108-7.
- ↑ Dennison, W. C.; O'Neil, J. M.; Duffy, E. J.; Oliver, P. E.; Shaw, G. R. (1999). "Blooms of the cyanobacterium Lyngbya majuscula in coastal waters of Queensland, Australia.". Bulletin de l’Institut océanographique. NS19 632: 501–506. Retrieved 2015-10-12.