Alkannin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
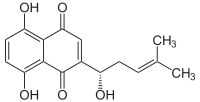
5,8-Dihydroxy-2-[(1S)-1-hydroxy-4-methylpent-3-en-1-yl]naphthalene-1,4-dione | |
| Other names
C.I. Natural red 20; Alkanet extract shikonin (±)-Alkannin (±)-Shikalkin (±)-Shikonin (±)-5,8-Dihydroxy-2-(1-hydroxy-4-methyl-3-pentenyl)-1,4-naphthoquinone | |
| Identifiers | |
| 517-88-4 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL28457 |
| ChemSpider | 65430 |
| ECHA InfoCard | 100.007.497 |
| E number | E103 (colours) |
| KEGG | C10292 |
| PubChem | 72521 |
| |
| |
| Properties[1] | |
| C 16H 16O 5 | |
| Molar mass | 288.29 g/mol |
| Appearance | Red-brown crystalline prisms |
| Density | 1.15 g/mL |
| Melting point | 149 °C (300 °F; 422 K) |
| Boiling point | 567 °C (1,053 °F; 840 K) |
| Sparingly soluble | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
3.0 g/kg (mice) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Alkannin is a natural dye that is obtained from the extracts of plants from the borage family Alkanna tinctoria that are found in the south of France. The dye is used as a food coloring and in cosmetics. It is used as a red-brown food additive in regions such as Australia,[2] and is designated in Europe as the E number E103, but is no longer approved for use.[3]
The chemical structure as a naphthoquinone derivative was first determined by Brockmann in 1936.[4] Alkannin has a deep red color in a greasy or oily environment and a violet color in an alkaline environment.
The enzyme 4-hydroxybenzoate geranyltransferase utilizes geranyl diphosphate and 4-hydroxybenzoate to produce 3-geranyl-4-hydroxybenzoate and diphosphate. These compounds are then used to form alkannin.[5]
Alkannin is an antioxidant[6] and has an antimicrobial effect against Staphylococcus aureus and Staphylococcus epidermidis. It is also known to have wound healing, antitumor, and antithrombotic properties.[5]
Shikonin is also found in the Chinese herbal medicine plant Lithospermum erythrorhizon, the red-root gromwell, (紫草 zicao, Pinyin: zǐcǎo). The dried root is a Chinese herbal medicine with various antiviral and biological activities, including inhibition of human immunodeficiency virus type 1 (HIV-1).[8][9][10]
References
- ↑ The Merck Index, 11th Edition, 243
- ↑ Additives, Food Standards Australia New Zealand
- ↑ "Current EU approved additives and their E Numbers", Food Standards Agency website, retrieved 15 Dec 2011
- ↑ H. Brockmann (1936). "Die Konstitution des Alkannins, Shikonins und Alkannans". Justus Liebigs Ann. Chem. 521: 1–47. doi:10.1002/jlac.19365210102.
- 1 2 Vassilios P. Papageorgiou; Andreana N. Assimopoulou; Elias A. Couladouros; David Hepworth; K. C. Nicolaou (1999). "The Chemistry and Biology of Alkannin, Shikonin, and Related Naphthazarin Natural Products". Angew. Chem. Int. Ed. 38 (3): 270–300. doi:10.1002/(SICI)1521-3773(19990201)38:3<270::AID-ANIE270>3.0.CO;2-0.
- ↑ A.N. Assimopoulou; D. Boskou; V.P. Papageorgiou (2004). "Antioxidant activities of alkannin, shikonin and Alkanna tinctoria root extracts in oil substrates". Food Chemistry. 87 (3): 433–438. doi:10.1016/j.foodchem.2003.12.017.
- ↑ Wiench, B; Eichhorn, T; Paulsen, M; Efferth, T (2012). "Shikonin Directly Targets Mitochondria and Causes Mitochondrial Dysfunction in Cancer Cells". Evidence-Based Complementary and Alternative Medicine. 2012: 726025. doi:10.1155/2012/726025. PMC 3478753
 . PMID 23118796.
. PMID 23118796. - ↑ Chen, X (Sep 2003). "Shikonin, a component of chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1.". Antimicrob Agents Chemother. 47 (9): 2810–6. doi:10.1128/aac.47.9.2810-2816.2003. PMID 12936978.
- ↑ Gao, H.; et al. (2011). "Anti-adenovirus activities of shikonin, a component of Chinese herbal medicine in vitro". Biol Pharm Bull. 34 (2): 197–202. doi:10.1248/bpb.34.197.
- ↑ Chen, J; Xie, J; Jiang, Z; Wang, B; Wang, Y; Hu, X (2011). "Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2.". Oncogene. 30: 4297–4306. doi:10.1038/onc.2011.137.