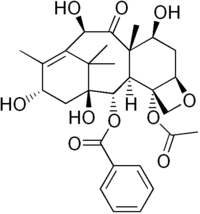
10-Deacetylbaccatin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-(Acetyloxy)-12-(benzoyloxy)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-dodecahydro-4,6,9,11-tetrahydroxy-4a,8,13,13-tetramethyl-7,11-methano-5H-cyclodeca(3,4)benz(1,2-b)oxet-5-one | |
| Other names
10-Deacetylbaccatin III 10-Deacetylbaccatine III | |
| Identifiers | |
| 32981-86-5 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:18193 |
| ChEMBL | ChEMBL393912 |
| ChemSpider | 135935 |
| ECHA InfoCard | 100.128.614 |
| PubChem | 154272 |
| |
| |
| Properties | |
| C29H36O10 | |
| Molar mass | 544.59 g/mol |
| Appearance | colorless solid |
| Melting point | 234 °C (453 °F; 507 K) |
| insoluble | |
| Solubility | soluble in methanol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
10-Deacetylbaccatins are a series of closely related natural organic compounds isolated from the Pacific yew tree (Taxus brevifolia) and related species. 10-Deacetylbaccatin III is a precursor to the anti-cancer drug docetaxel (Taxotere).
10-deacetylbaccatin III 10-O-acetyltransferase converts 10-deacetylbaccatin to baccatin III:
- acetyl-CoA + 10-deacetylbaccatin III ⇌ CoA + baccatin III
External links
- CID 6712096 from PubChem - 7-Epi-10-Deacetylbaccatin III
- CID 9872117 from PubChem - 10-Deacetylbaccatin V
- CID 5316348 from PubChem - 10-Deacetylbaccatin VI
- CID 5317065 from PubChem - 13-Epi-10-Deacetylbaccatin III
- CID 15957452 from PubChem - 13-[3-(2-Naphthyl)prop-2-enoyl]-2-debenzoyl-2-(4-methoxybenzoxyl)-10-deacetylbaccatin III
- CID 15957458 from PubChem - 10-N,N-Dimethylglycyl-13-[3-(2-Naphthyl)prop-2-enoyl]-10-deacetylbaccatin III
This article is issued from Wikipedia - version of the 11/1/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.